Einkorn genomics sheds light on history of the oldest domesticated wheat
- PMID: 37532937
- PMCID: PMC10447253
- DOI: 10.1038/s41586-023-06389-7
Einkorn genomics sheds light on history of the oldest domesticated wheat
Abstract
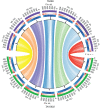
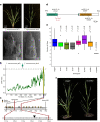
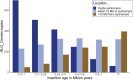
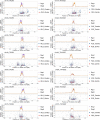
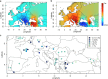
Einkorn (Triticum monococcum) was the first domesticated wheat species, and was central to the birth of agriculture and the Neolithic Revolution in the Fertile Crescent around 10,000 years ago1,2. Here we generate and analyse 5.2-Gb genome assemblies for wild and domesticated einkorn, including completely assembled centromeres. Einkorn centromeres are highly dynamic, showing evidence of ancient and recent centromere shifts caused by structural rearrangements. Whole-genome sequencing analysis of a diversity panel uncovered the population structure and evolutionary history of einkorn, revealing complex patterns of hybridizations and introgressions after the dispersal of domesticated einkorn from the Fertile Crescent. We also show that around 1% of the modern bread wheat (Triticum aestivum) A subgenome originates from einkorn. These resources and findings highlight the history of einkorn evolution and provide a basis to accelerate the genomics-assisted improvement of einkorn and bread wheat.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

