TDP-43 forms amyloid filaments with a distinct fold in type A FTLD-TDP
- PMID: 37532939
- PMCID: PMC10447236
- DOI: 10.1038/s41586-023-06405-w
TDP-43 forms amyloid filaments with a distinct fold in type A FTLD-TDP
Abstract
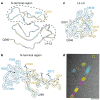
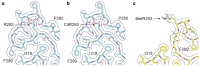
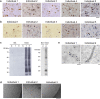
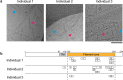
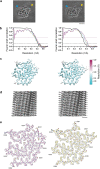
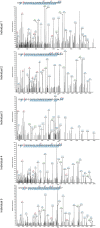
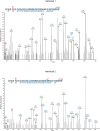
The abnormal assembly of TAR DNA-binding protein 43 (TDP-43) in neuronal and glial cells characterizes nearly all cases of amyotrophic lateral sclerosis (ALS) and around half of cases of frontotemporal lobar degeneration (FTLD)1,2. A causal role for TDP-43 assembly in neurodegeneration is evidenced by dominantly inherited missense mutations in TARDBP, the gene encoding TDP-43, that promote assembly and give rise to ALS and FTLD3-7. At least four types (A-D) of FTLD with TDP-43 pathology (FTLD-TDP) are defined by distinct brain distributions of assembled TDP-43 and are associated with different clinical presentations of frontotemporal dementia8. We previously showed, using cryo-electron microscopy, that TDP-43 assembles into amyloid filaments in ALS and type B FTLD-TDP9. However, the structures of assembled TDP-43 in FTLD without ALS remained unknown. Here we report the cryo-electron microscopy structures of assembled TDP-43 from the brains of three individuals with the most common type of FTLD-TDP, type A. TDP-43 formed amyloid filaments with a new fold that was the same across individuals, indicating that this fold may characterize type A FTLD-TDP. The fold resembles a chevron badge and is unlike the double-spiral-shaped fold of ALS and type B FTLD-TDP, establishing that distinct filament folds of TDP-43 characterize different neurodegenerative conditions. The structures, in combination with mass spectrometry, led to the identification of two new post-translational modifications of assembled TDP-43, citrullination and monomethylation of R293, and indicate that they may facilitate filament formation and observed structural variation in individual filaments. The structures of TDP-43 filaments from type A FTLD-TDP will guide mechanistic studies of TDP-43 assembly, as well as the development of diagnostic and therapeutic compounds for TDP-43 proteinopathies.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Comment in
-
New mechanistic insights into TDP-43 pathology.Nat Rev Neurol. 2023 Oct;19(10):574. doi: 10.1038/s41582-023-00870-7. Nat Rev Neurol. 2023. PMID: 37608120 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

