Development of an mRNA-lipid nanoparticle vaccine against Lyme disease
- PMID: 37533256
- PMCID: PMC10492027
- DOI: 10.1016/j.ymthe.2023.07.022
Development of an mRNA-lipid nanoparticle vaccine against Lyme disease
Abstract
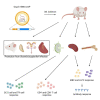
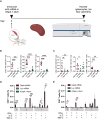
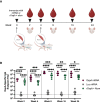
Lyme disease is the most common vector-borne infectious disease in the United States, in part because a vaccine against it is not currently available for humans. We propose utilizing the lipid nanoparticle-encapsulated nucleoside-modified mRNA (mRNA-LNP) platform to generate a Lyme disease vaccine like the successful clinical vaccines against SARS-CoV-2. Of the antigens expressed by Borrelia burgdorferi, the causative agent of Lyme disease, outer surface protein A (OspA) is the most promising candidate for vaccine development. We have designed and synthesized an OspA-encoding mRNA-LNP vaccine and compared its immunogenicity and protective efficacy to an alum-adjuvanted OspA protein subunit vaccine. OspA mRNA-LNP induced superior humoral and cell-mediated immune responses in mice after a single immunization. These potent immune responses resulted in protection against bacterial infection. Our study demonstrates that highly efficient mRNA vaccines can be developed against bacterial targets.
Keywords: Borrelia burgdorferi; Lyme disease; OspA; antibody; antigen; bacteria; lipid nanoparticle; mRNA; nucleoside-modification; spirochete; vaccine.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests In accordance with the University of Pennsylvania policies and procedures and our ethical obligations as researchers, we report that D.W. is named on patents that describe the use of nucleoside-modified mRNA as a platform to deliver therapeutic proteins. N.P., D.W., and Y.K.T. are named on a patent describing the use of nucleoside-modified mRNA in lipid nanoparticles as a vaccine platform. We have disclosed those interests fully to the University of Pennsylvania, and we have in place an approved plan for managing any potential conflicts arising from licensing of our patents. Y.K.T. is an employee of Acuitas Therapeutics, a company focused on the development of LNP nucleic acid delivery systems for therapeutic applications. N.P. served on the mRNA strategic advisory board of Sanofi Pasteur in 2022. N.P. is a member of the scientific advisory board of AldexChem.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

