Treatment of inflammatory bowel disease-Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn's and Colitis meeting
- PMID: 37533265
- PMCID: PMC10397553
- DOI: 10.5217/ir.2022.00135
Treatment of inflammatory bowel disease-Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn's and Colitis meeting
Abstract
Background/aims: As the characteristics of inflammatory bowel disease (IBD) differ between Asians and Westerners, it is necessary to determine adequate therapeutic strategy for Asian IBD patients. We evaluated the current treatment of IBD in Asian countries/regions using a web-based survey.
Methods: The Korean Association for the Study of Intestinal Diseases conducted a multinational web-based survey for current IBD care in Asia between September 16, 2020, and November 13, 2020.
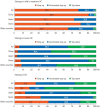
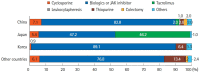
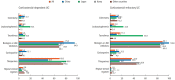
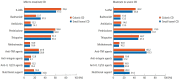
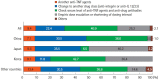
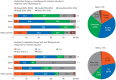
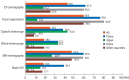
Results: A total of 384 doctors treating IBD patients from 24 Asian countries/regions responded to the survey. Anti-tumor necrosis factor (TNF) agents, anti-integrins, and anti-interleukin-12/23 agents were available for use by 93.8%, 72.1%, and 70.1% of respondents in Asian countries/regions. Compared with a previous survey performed in 2014, an increased tendency for treatment with biologics, including anti-TNF agents, was observed. In the treatment of corticosteroid-refractory acute severe ulcerative colitis, 72.1% of respondents chose anti-TNF agents, followed by tacrolimus (11.7%). In the treatment of corticosteroid-refractory Crohn's disease, 90.4% chose anti-TNF agents, followed by thiopurines (53.1%), anti-interleukin-12/23 agents (39.3%), and anti-integrin agents (35.7%). In the treatment of Crohn's disease patients refractory to anti-TNF agents, the most preferred strategy was to measure serum levels of anti-TNF and anti-drug antibodies (40.9%), followed by empiric dose escalation or shortening of dosing intervals (25.3%).
Conclusions: Although there were some differences, treatment strategies for patients with IBD were mostly similar among Asian doctors. Based on the therapeutic outcomes, it is necessary to identify the most appropriate therapeutic strategy for Asian IBD patients.
Keywords: Asia; Inflammatory bowel diseases; Survey; Treatment.
Conflict of interest statement
Ng SC has served as a speaker for Janssen, Abbvie, Takeda, Ferring, Tillotts, Menarini, Pfizer and has received research grants from Olympus, Ferring, Janssen and Abbvie. She is scientific cofounder of GenieBiome Limited and sits on the Board of Directors of GenieBiome Ltd. Hisamatsu T has performed joint research with Alfresa Pharma Co., Ltd., and EA Pharma Co., Ltd., received grant support from Mitsubishi Tanabe Pharma Corporation, EA Pharma Co., Ltd., AbbVie GK, JIMRO Co., Ltd., Zeria Pharmaceutical Co., Ltd., Daiichi-Sankyo, Kyorin Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Inc., and Mochida Pharmaceutical Co., Ltd., and received consulting and lecture fees from Mitsubishi Tanabe Pharma Corporation, AbbVie GK, Celgene K.K., EA Pharma Co., Ltd., Kyorin Pharmaceutical Co., Ltd., JIMRO Co., Janssen Pharmaceutical K.K., Mochida Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Pfizer Inc. Ye BD has served on advisory boards for AbbVie Korea, Celltrion, Daewoong Pharma, Ferring Korea, Janssen Korea, Pfizer Korea, Takeda, and Takeda Korea, has received research grants from Celltrion and Pfizer Korea, has received consulting fees from BMS Pharmaceutical Korea Ltd., Chong Kun Dang Pharm., CJ Red BIO, Curacle, Daewoong Pharma, IQVIA, Kangstem Biotech, Korea Otsuka Pharm, Korea United Pharm. Inc., Medtronic Korea, NanoEntek, ORGANOIDSCIENCES LTD., and Takeda, and has received speaker fees from AbbVie Korea, Celltrion, Cornerstones Health, Curacle, Daewoong Pharma, Ferring Korea, IQVIA, Janssen Korea, Pfizer Korea, Takeda, and Takeda Korea. Except for that, no potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Treatment of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn's and Colitis (AOCC) meeting in Seoul.Intest Res. 2016 Jul;14(3):231-9. doi: 10.5217/ir.2016.14.3.231. Epub 2016 Jun 27. Intest Res. 2016. PMID: 27433145 Free PMC article.
-
Diagnosis of inflammatory bowel disease-Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn's and Colitis meeting.Intest Res. 2023 Jul;21(3):328-338. doi: 10.5217/ir.2023.00012. Epub 2023 Jul 27. Intest Res. 2023. PMID: 37533264 Free PMC article.
-
Diagnosis of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization for Crohn's and Colitis (AOCC) meeting in Seoul.Intest Res. 2016 Jul;14(3):224-30. doi: 10.5217/ir.2016.14.3.224. Epub 2016 Jun 27. Intest Res. 2016. PMID: 27433144 Free PMC article.
-
Asian Organization for Crohn's and Colitis and Asian Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: Risk assessment.J Gastroenterol Hepatol. 2018 Jan;33(1):20-29. doi: 10.1111/jgh.14019. J Gastroenterol Hepatol. 2018. PMID: 29023903 Review.
-
Asian Organization for Crohn's and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 2: Management.J Gastroenterol Hepatol. 2018 Jan;33(1):30-36. doi: 10.1111/jgh.14018. J Gastroenterol Hepatol. 2018. PMID: 29024102 Review.
Cited by
-
How have treatment patterns for patients with inflammatory bowel disease changed in Asian countries?Intest Res. 2023 Jul;21(3):275-276. doi: 10.5217/ir.2023.00061. Epub 2023 Jul 27. Intest Res. 2023. PMID: 37533261 Free PMC article. No abstract available.
-
Optimizing Infliximab Use in Real-World Inflammatory Bowel Disease: Insights from a Population Pharmacokinetic Model Integrating Intravenous and Subcutaneous Formulations.Gut Liver. 2025 May 15;19(3):299-300. doi: 10.5009/gnl250154. Gut Liver. 2025. PMID: 40356324 Free PMC article. No abstract available.
-
Role of 5-aminosalicylic acid in ulcerative colitis management in 8 Asian territories: a physician survey.Intest Res. 2025 Apr;23(2):117-128. doi: 10.5217/ir.2024.00085. Epub 2025 Jan 6. Intest Res. 2025. PMID: 39757455 Free PMC article.
-
Inflammatory bowel disease: a narrative review of disease evolution in South Asia and India over the last decade.Therap Adv Gastroenterol. 2024 Nov 20;17:17562848241258360. doi: 10.1177/17562848241258360. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 39575157 Free PMC article. Review.
-
Enrichment of Activated Fibroblasts as a Potential Biomarker for a Non-Durable Response to Anti-Tumor Necrosis Factor Therapy in Patients with Crohn's Disease.Int J Mol Sci. 2023 Sep 30;24(19):14799. doi: 10.3390/ijms241914799. Int J Mol Sci. 2023. PMID: 37834250 Free PMC article.
References
-
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. - PubMed
-
- Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: east meets west. J Gastroenterol Hepatol. 2020;35:380–389. - PubMed
-
- Ng SC, Leung WK, Shi HY, et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a territory-wide population-based registry in Hong Kong. Inflamm Bowel Dis. 2016;22:1954–1960. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

