Safety and immunogenicity of a variant-adapted SARS-CoV-2 recombinant protein vaccine with AS03 adjuvant as a booster in adults primed with authorized vaccines: a phase 3, parallel-group study
- PMID: 37533419
- PMCID: PMC10391925
- DOI: 10.1016/j.eclinm.2023.102109
Safety and immunogenicity of a variant-adapted SARS-CoV-2 recombinant protein vaccine with AS03 adjuvant as a booster in adults primed with authorized vaccines: a phase 3, parallel-group study
Abstract
Background: In a parallel-group, international, phase 3 study (ClinicalTrials.govNCT04762680), we evaluated prototype (D614) and Beta (B.1.351) variant recombinant spike protein booster vaccines with AS03-adjuvant (CoV2 preS dTM-AS03).
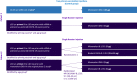
Methods: Adults, previously primed with mRNA (BNT162b2, mRNA-1273), adenovirus-vectored (Ad26.CoV2.S, ChAdOx1nCoV-19) or protein (CoV2 preS dTM-AS03 [monovalent D614; MV(D614)]) vaccines were enrolled between 29 July 2021 and 22 February 2022. Participants were stratified by age (18-55 and ≥ 56 years) and received one of the following CoV2 preS dTM-AS03 booster formulations: MV(D614) (n = 1285), MV(B.1.351) (n = 707) or bivalent D614 + B.1.351 (BiV; n = 625). Unvaccinated adults who tested negative on a SARS-CoV-2 rapid diagnostic test (control group, n = 479) received two primary doses, 21 days apart, of MV(D614). Anti-D614G and anti-B.1.351 antibodies were evaluated using validated pseudovirus (lentivirus) neutralization (PsVN) assay 14 days post-booster (day [D]15) in 18-55-year-old BNT162b2-primed participants and compared with those pre-booster (D1) and on D36 in 18-55-year-old controls (primary immunogenicity endpoints). PsVN titers to Omicron BA.1, BA.2 and BA.4/5 subvariants were also evaluated. Safety was evaluated over a 12-month follow-up period. Planned interim analyses are presented up to 14 days post-last vaccination for immunogenicity and over a median duration of 5 months for safety.
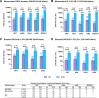
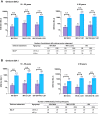
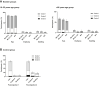
Findings: All three boosters elicited robust anti-D614G or -B.1.351 PsVN responses for mRNA, adenovirus-vectored and protein vaccine-primed groups. Among BNT162b2-primed adults (18-55 years), geometric means of the individual post-booster versus pre-booster titer ratio (95% confidence interval [CI]) were: for MV (D614), 23.37 (18.58-29.38) (anti-D614G); for MV(B.1.351), 35.41 (26.71-46.95) (anti-B.1.351); and for BiV, 14.39 (11.39-18.28) (anti-D614G) and 34.18 (25.84-45.22 (anti-B.1.351). GMT ratios (98.3% CI) versus post-primary vaccination GMTs in controls, were: for MV(D614) booster, 2.16 (1.69; 2.75) [anti-D614G]; for MV(B.1.351), 1.96 (1.54; 2.50) [anti-B.1.351]; and for BiV, 2.34 (1.84; 2.96) [anti-D614G] and 1.39 (1.09; 1.77) [anti-B.1.351]. All booster formulations elicited cross-neutralizing antibodies against Omicron BA.2 (across priming vaccine subgroups), Omicron BA.1 (BNT162b2-primed participants) and Omicron BA.4/5 (BNT162b2-primed participants and MV D614-primed participants). Similar patterns in antibody responses were observed for participants aged ≥56 years. Reactogenicity tended to be transient and mild-to-moderate severity in all booster groups. No safety concerns were identified.
Interpretation: CoV2 preS dTM-AS03 boosters demonstrated acceptable safety and elicited robust neutralizing antibodies against multiple variants, regardless of priming vaccine.
Funding: Sanofi and Biomedical Advanced Research and Development Authority (BARDA).
Keywords: AS03 adjuvant; B.1.351; Beta; Booster; COVID-19; CoV2 preS dTM-AS03; Immunogenicity; Omicron; Recombinant protein vaccine; SARS-CoV-2; Safety.
© 2023 The Author(s).
Conflict of interest statement
GdB, JW, AP, MSR, HA, MIB, RMCa, RF, BF, JG, M-HG, SG, OH, RM, JP, NR, RMCh and SSr are Sanofi employees. GdB, AP, MIB, BF, OH, NR, RMCh and SSr hold stock or stock options in Sanofi. SSa was a Sanofi employee at the time of study conduct and held shares and/or stock options in the company at the time of study conduct. GdB, SSr, RMCh, and SSa are inventors on a pending patent application filed by Sanofi and GSK for the development of the CoV-2 dTM vaccine. MAC, LS and MK are employed by GSK and hold restricted shares in the GSK group of companies. FTDS was employed by GSK and held restricted shares in the GSK group of companies, at the time of the study. FMT declares trial fees paid to their institution by Sanofi; honoraria received from GSK group of companies, Pfizer Inc., Sanofi, MSD, Moderna, Biofabri, AstraZeneca, Novavax, Janssen; meeting and/or travel fees received from Pfizer Inc, MSD, GSK and Sanofi; data safety monitoring board or advisory board participation for Pfizer and Biofabri; being a member of ETAGE–WHO Europe, coordinator of the Spanish Pediatric Critical Trials Network and coordinator of the WHO Collaborating Centre for Vaccines Safety of Santiago de Compostela; and payments made to their institution for their role as principal investigator in randomized controlled trials for Ablynx, Abbot, Seqirus, Sanofi, MSD, Merck, Pfizer, Roche, Regeneron, Janssen, Medimmune, Novavax, Novartis and GSK. DMRM declares that her institution received funding from Sanofi. AF receives research funding, paid to his employers, from Sanofi both for work related to this study and other unrelated vaccine trials and from GSK for other unrelated studies. He receives research funding from other vaccine manufacturers relating to trials and studies and undertakes paid consultancy related to a number of developmental antimicrobial drugs and vaccines. OL declares that their institution received funding for conducting the trial. NLM received travel support from Sanofi and chaired a Scientific Advisory Board for them, unrelated to current study. LDS received a research grant from Sanofi. SD, SA, DB, AB, DD, PG, JAH, CR and AW declare no conflicts of interest.
Figures

Similar articles
-
Safety and immunogenicity of an AS03-adjuvanted SARS-CoV-2 recombinant protein vaccine (CoV2 preS dTM) in healthy adults: interim findings from a phase 2, randomised, dose-finding, multicentre study.Lancet Infect Dis. 2022 May;22(5):636-648. doi: 10.1016/S1473-3099(21)00764-7. Epub 2022 Jan 25. Lancet Infect Dis. 2022. PMID: 35090638 Free PMC article. Clinical Trial.
-
Beta-variant recombinant SARS CoV-2 vaccine induces durable cross-reactive antibodies against Omicron BA variants.Commun Med (Lond). 2025 Jan 18;5(1):21. doi: 10.1038/s43856-024-00675-9. Commun Med (Lond). 2025. PMID: 39827332 Free PMC article.
-
Immunogenicity of bivalent omicron (BA.1) booster vaccination after different priming regimens in health-care workers in the Netherlands (SWITCH ON): results from the direct boost group of an open-label, multicentre, randomised controlled trial.Lancet Infect Dis. 2023 Aug;23(8):901-913. doi: 10.1016/S1473-3099(23)00140-8. Epub 2023 Apr 21. Lancet Infect Dis. 2023. PMID: 37088096 Free PMC article. Clinical Trial.
-
Post-Vaccination Neutralization Responses to Omicron Sub-Variants.Vaccines (Basel). 2022 Oct 20;10(10):1757. doi: 10.3390/vaccines10101757. Vaccines (Basel). 2022. PMID: 36298622 Free PMC article. Review.
-
The immunologic outcomes and adverse events of COVID-19 vaccine booster dose in immunosuppressed people: A systematic review.Prev Med Rep. 2024 May 31;44:102778. doi: 10.1016/j.pmedr.2024.102778. eCollection 2024 Aug. Prev Med Rep. 2024. PMID: 38979481 Free PMC article. Review.
Cited by
-
COVID-19 Vaccines: Where Did We Stand at the End of 2023?Viruses. 2024 Jan 29;16(2):203. doi: 10.3390/v16020203. Viruses. 2024. PMID: 38399979 Free PMC article. Review.
-
Beta-containing bivalent SARS-CoV-2 protein vaccine elicits durable broad neutralization in macaques and protection in hamsters.Commun Med (Lond). 2023 May 26;3(1):75. doi: 10.1038/s43856-023-00302-z. Commun Med (Lond). 2023. PMID: 37237062 Free PMC article.
-
Beta-variant recombinant booster vaccine elicits broad cross-reactive neutralization of SARS-CoV-2 including Omicron variants.Heliyon. 2024 Feb 28;10(5):e27033. doi: 10.1016/j.heliyon.2024.e27033. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38486776 Free PMC article.
-
Transforming vaccinology.Cell. 2024 Sep 19;187(19):5171-5194. doi: 10.1016/j.cell.2024.07.021. Cell. 2024. PMID: 39303685 Review.
-
Navigating the Landscape of B Cell Mediated Immunity and Antibody Monitoring in SARS-CoV-2 Vaccine Efficacy: Tools, Strategies and Clinical Trial Insights.Vaccines (Basel). 2024 Sep 24;12(10):1089. doi: 10.3390/vaccines12101089. Vaccines (Basel). 2024. PMID: 39460256 Free PMC article. Review.
References
-
- World Health Organization WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/ Available at:
-
- World Health Organization Interim statement on decision-making considerations for the use of variant updated COVID-19 vaccines. 2022. https://www.who.int/news/item/17-06-2022-interim-statement-on-decision-m... Available at:
-
- Ferdinands J.M., Rao S., Dixon B.E., et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION network, 10 states, august 2021-january 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–263. - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

