CETP inhibitor evacetrapib enters mouse brain tissue
- PMID: 37533630
- PMCID: PMC10390775
- DOI: 10.3389/fphar.2023.1171937
CETP inhibitor evacetrapib enters mouse brain tissue
Abstract
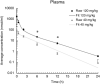
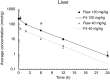
High levels of plasma cholesterol, especially high levels of low-density lipoprotein cholesterol (LDL-C), have been associated with an increased risk of Alzheimer's disease. The cholesteryl ester transfer protein (CETP) in plasma distributes cholesteryl esters between lipoproteins and increases LDL-C in plasma. Epidemiologically, decreased CETP activity has been associated with sustained cognitive performance during aging, longevity, and a lower risk of Alzheimer's disease. Thus, pharmacological CETP inhibitors could be repurposed for the treatment of Alzheimer's disease as they are safe and effective at lowering CETP activity and LDL-C. Although CETP is mostly expressed by the liver and secreted into the bloodstream, it is also expressed by astrocytes in the brain. Therefore, it is important to determine whether CETP inhibitors can enter the brain. Here, we describe the pharmacokinetic parameters of the CETP inhibitor evacetrapib in the plasma, liver, and brain tissues of CETP transgenic mice. We show that evacetrapib crosses the blood-brain barrier and is detectable in brain tissue 0.5 h after a 40 mg/kg i.v. injection in a non-linear function. We conclude that evacetrapib may prove to be a good candidate to treat CETP-mediated cholesterol dysregulation in Alzheimer's disease.
Keywords: Alzheimer’s disease; PBPK model; brain; cholesterol; cholesteryl ester transfer protein (CETP); evacetrapib; inhibitor; pharmacokinetic.
Copyright © 2023 Phénix, Côté, Dieme, Recinto, Oestereich, Efrem, Haddad, Bouchard and Munter.
Conflict of interest statement
LM received funds from New Amsterdam Pharma for a research project regarding CETP inhibitors independent from the work presented herein. This PK study was completed in its core prior to this funding. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Effects of the cholesteryl ester transfer protein inhibitor evacetrapib on lipoproteins, apolipoproteins and 24-h ambulatory blood pressure in healthy adults.J Pharm Pharmacol. 2014 Nov;66(11):1576-85. doi: 10.1111/jphp.12287. Epub 2014 Jun 24. J Pharm Pharmacol. 2014. PMID: 24961753 Free PMC article. Clinical Trial.
-
Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure.J Lipid Res. 2011 Dec;52(12):2169-2176. doi: 10.1194/jlr.M018069. Epub 2011 Sep 25. J Lipid Res. 2011. PMID: 21957197 Free PMC article.
-
Cholesteryl ester transfer protein inhibitors: from high-density lipoprotein cholesterol to low-density lipoprotein cholesterol lowering agents?Cardiovasc Res. 2022 Nov 10;118(14):2919-2931. doi: 10.1093/cvr/cvab350. Cardiovasc Res. 2022. PMID: 34849601 Free PMC article.
-
Evacetrapib: Another CETP Inhibitor for Dyslipidemia With No Clinical Benefit.Cardiol Rev. 2017 Mar/Apr;25(2):43-52. doi: 10.1097/CRD.0000000000000137. Cardiol Rev. 2017. PMID: 28099220 Review.
-
On- and off-target pharmacology of torcetrapib: current understanding and implications for the structure activity relationships (SAR), discovery and development of cholesteryl ester-transfer protein (CETP) inhibitors.Drugs. 2012 Mar 5;72(4):491-507. doi: 10.2165/11599310-000000000-00000. Drugs. 2012. PMID: 22356288 Review.
Cited by
-
Roles of peripheral lipoproteins and cholesteryl ester transfer protein in the vascular contributions to cognitive impairment and dementia.Mol Neurodegener. 2023 Nov 16;18(1):86. doi: 10.1186/s13024-023-00671-y. Mol Neurodegener. 2023. PMID: 37974180 Free PMC article. Review.
-
Cholesteryl ester transfer protein inhibition: a pathway to reducing risk of morbidity and promoting longevity.Curr Opin Lipidol. 2024 Dec 1;35(6):303-309. doi: 10.1097/MOL.0000000000000955. Epub 2024 Oct 17. Curr Opin Lipidol. 2024. PMID: 39508067 Free PMC article. Review.
-
The Pleiotropic Effects of Lipid-Modifying Interventions: Exploring Traditional and Emerging Hypolipidemic Therapies.Metabolites. 2024 Jul 17;14(7):388. doi: 10.3390/metabo14070388. Metabolites. 2024. PMID: 39057711 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

