Ultrasonic-Assisted Synthesis of Heterocyclic Curcumin Analogs as Antidiabetic, Antibacterial, and Antioxidant Agents Combined with in vitro and in silico Studies
- PMID: 37533689
- PMCID: PMC10392906
- DOI: 10.2147/AABC.S403413
Ultrasonic-Assisted Synthesis of Heterocyclic Curcumin Analogs as Antidiabetic, Antibacterial, and Antioxidant Agents Combined with in vitro and in silico Studies
Abstract
Background: Heterocyclic analogs of curcumin have a wide range of therapeutic potential and the ability to control the activity of a variety of metabolic enzymes.
Methods: 1H-NMR and 13C-NMR spectroscopic techniques were used to determine the structures of synthesized compounds. The agar disc diffusion method and α-amylase inhibition assay were used to examine the antibacterial and anti-diabetic potential of the compounds against α-amylase enzyme inhibitory activity, respectively. DPPH-free radical scavenging and lipid peroxidation inhibition assays were used to assess the in vitro antioxidant potential.
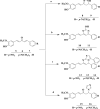
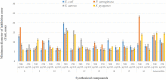
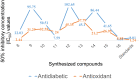
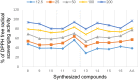
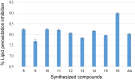
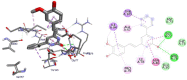
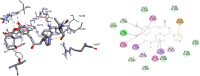
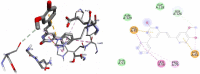
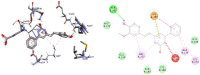
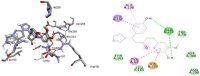
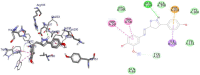
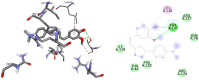
Results and discussion: In this work, nine heterocyclic analogs derived from curcumin precursors under ultrasonic irradiation were synthesized in excellent yields (81.4-93.7%) with improved reaction time. Results of antibacterial activities revealed that compounds 8, and 11 displayed mean inhibition zone of 13.00±0.57, and 19.66±00 mm, respectively, compared to amoxicillin (12.87±1.41 mm) at 500 μg/mL against E. coli, while compounds 8, 11 and 16 displayed mean inhibition zone of 17.67±0.57, 14.33±0.57 and 23.33±00 mm, respectively, compared to amoxicillin (13.75±1.83 mm) at 500 μg/mL against P. aeruginosa. Compound 11 displayed a mean inhibition zone of 11.33±0.57 mm compared to amoxicillin (10.75±1.83 mm) at 500 μg/mL against S. aureus. Compound 11 displayed higher binding affinities of -7.5 and -8.3 Kcal/mol with penicillin-binding proteins (PBPs) and β-lactamases producing bacterial strains, compared to amoxicillin (-7.2 and -7.9 Kcal/mol, respectively), these results are in good agreement with the in vitro antibacterial activities. In vitro antidiabetic potential on α-amylase enzyme revealed that compounds 11 (IC50=7.59 µg/mL) and 16 (IC50=4.08 µg/mL) have higher inhibitory activities than acarbose (IC50=8.0 µg/mL). Compound 8 showed promising antioxidant inhibition efficacy of DPPH (IC50 = 2.44 g/mL) compared to ascorbic acid (IC50=1.24 g/mL), while compound 16 revealed 89.9±20.42% inhibition of peroxide generation showing its potential in reducing the development of lipid peroxides. In silico molecular docking analysis, results are in good agreement with in vitro biological activity. In silico ADMET profiles suggested the adequate oral drug-likeness potential of the compounds without adverse effects.
Conclusion: According to our findings, both biological activities and in silico computational studies results demonstrated that compounds 8, 11, and 16 are promising α-amylase inhibitors and antibacterial agents against E. coli, P. aeruginosa, and S. aureus, whereas compound 8 was found to be a promising antioxidant agent.
Keywords: ADMET prediction; antibacterial; antioxidant; heterocyclic curcumin analogs; molecular docking; ultrasonic-irradiation; α-amylase.
© 2023 Zelelew et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures
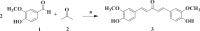
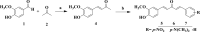

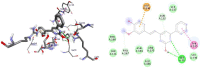
References
-
- World Health Organization. Noncommunicable diseases country profiles, 2018; 2018.
LinkOut - more resources
Full Text Sources

