Multivalent vaccines demonstrate immunogenicity and protect against Coxiella burnetii aerosol challenge
- PMID: 37533862
- PMCID: PMC10390735
- DOI: 10.3389/fimmu.2023.1192821
Multivalent vaccines demonstrate immunogenicity and protect against Coxiella burnetii aerosol challenge
Abstract
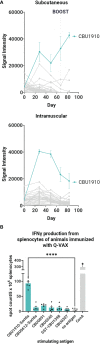
Vaccines are among the most cost-effective public health measures for controlling infectious diseases. Coxiella burnetii is the etiological agent of Q fever, a disease with a wide clinical spectrum that ranges from mild symptoms, such as fever and fatigue, to more severe disease, such as pneumonia and endocarditis. The formalin-inactivated whole-cell vaccine Q-VAX® contains hundreds of antigens and confers lifelong protection in humans, but prior sensitization from infection or vaccination can result in deleterious reactogenic responses to vaccination. Consequently, there is great interest in developing non-reactogenic alternatives based on adjuvanted recombinant proteins. In this study, we aimed to develop a multivalent vaccine that conferred protection with reduced reactogenicity. We hypothesized that a multivalent vaccine consisting of multiple antigens would be more immunogenic and protective than a monovalent vaccine owing to the large number of potential protective antigens in the C. burnetii proteome. To address this, we identified immunogenic T and B cell antigens, and selected proteins were purified to evaluate with a combination adjuvant (IVAX-1), with or without C. burnetii lipopolysaccharide (LPS) in immunogenicity studies in vivo in mice and in a Hartley guinea pig intratracheal aerosol challenge model using C. burnetii strain NMI RSA 493. The data showed that multivalent vaccines are more immunogenic than monovalent vaccines and more closely emulate the protection achieved by Q-VAX. Although six antigens were the most immunogenic, we also discovered that multiplexing beyond four antigens introduces detectable reactogenicity, indicating that there is an upper limit to the number of antigens that can be safely included in a multivalent Q-fever vaccine. C. burnetii LPS also demonstrates efficacy as a vaccine antigen in conferring protection in an otherwise monovalent vaccine formulation, suggesting that its addition in multivalent vaccines, as demonstrated by a quadrivalent formulation, would improve protective responses.
Keywords: Coxiella burnetii; adjuvant; aerosol challenge; guinea pig; hypersensitivity; multivalency; reactogenicity; subunit vaccine.
Copyright © 2023 Jan, Fratzke, Felgner, Hernandez-Davies, Liang, Nakajima, Jasinskas, Supnet, Jain, Felgner, Davies and Gregory.
Conflict of interest statement
SJ, JF, JH-D, LL, RN, AJas, AJai, PF, and DHD own shares in MyImmunome Inc. MyImmunome does not sell any products described in this paper, nor funded any part of the work described herein. Neither MyImmunome or its shareholders are likely to benefit from the results described in this publication. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Chiu CK, Durrheim DN. A review of the efficacy of human q fever vaccine registered in Australia. In: Database of abstracts of reviews of effects (DARE): quality-assessed reviews. UK: Centre for Reviews and Dissemination; (2007). Available at: https://www.ncbi.nlm.nih.gov/books/NBK74583/. - PubMed

