Characterization of the humoral and cellular immunity induced by a recombinant BCG vaccine for the respiratory syncytial virus in healthy adults
- PMID: 37533867
- PMCID: PMC10390696
- DOI: 10.3389/fimmu.2023.1215893
Characterization of the humoral and cellular immunity induced by a recombinant BCG vaccine for the respiratory syncytial virus in healthy adults
Abstract
Introduction: The human respiratory syncytial virus (hRSV) is responsible for most respiratory tract infections in infants. Even though currently there are no approved hRSV vaccines for newborns or infants, several candidates are being developed. rBCG-N-hRSV is a vaccine candidate previously shown to be safe in a phase I clinical trial in adults (clinicaltrials.gov identifier #NCT03213405). Here, secondary immunogenicity analyses were performed on these samples.
Methods: PBMCs isolated from immunized volunteers were stimulated with hRSV or mycobacterial antigens to evaluate cytokines and cytotoxic T cell-derived molecules and the expansion of memory T cell subsets. Complement C1q binding and IgG subclass composition of serum antibodies were assessed.
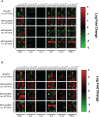
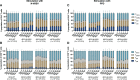
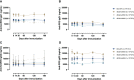
Results: Compared to levels detected prior to vaccination, perforin-, granzyme B-, and IFN-γ-producing PBMCs responding to stimulus increased after immunization, along with their effector memory response. N-hRSV- and mycobacterial-specific antibodies from rBCG-N-hRSV-immunized subjects bound C1q.
Conclusion: Immunization with rBCG-N-hRSV induces cellular and humoral immune responses, supporting that rBCG-N-hRSV is immunogenic and safe in healthy individuals.
Clinical trial registration: https://classic.clinicaltrials.gov/ct2/show/, identifier NCT03213405.
Keywords: BCG; cellular response; clinical trial; hRSV; vaccine.
Copyright © 2023 Pacheco, Andrade, Gálvez, Vázquez, Rodríguez-Guilarte, Abarca, González, Bueno and Kalergis.
Conflict of interest statement
PG, SB, and AK hold a patent for the rBCG-N-hRSV vaccine with Pontificia Universidad Católica de Chile PCT/US2008/076682. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. . Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet (2010) 375:1545–55. doi: 10.1016/S0140-6736(10)60206-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

