Shortening identification times: comparative observational study of three early blood culture testing protocols
- PMID: 37533929
- PMCID: PMC10390722
- DOI: 10.3389/fcimb.2023.1192002
Shortening identification times: comparative observational study of three early blood culture testing protocols
Abstract
Background: While early appropriate antibiotic therapy is a proven means of limiting the progression of infections, especially bacteremia, empirical antibiotic therapy in sepsis is ineffective up to 30%. The aim of this study was to compare early blood culture testing protocols in terms of their ability to shorten the delay between blood sampling and appropriate antibiotic therapy.
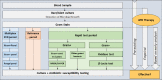
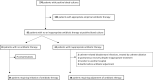
Methods: In this french observational study, we compared three blood culture testing protocols. Positive blood cultures were tested using either GenMark ePlex panels (multiplex PCR period), a combination of MRSA/SA PCR, β-Lacta and oxidase tests (multitest period), or conventional identification and susceptibility tests only (reference period). Conventional identification and susceptibility tests were performed in parallel for all samples, as the gold standard.
Results: Among the 270 patients with positive blood cultures included, early and conventional results were in good agreement, especially for the multitest period. The delay between a blood culture positivity and initial results was 3.8 (2.9-6.9) h in the multiplex PCR period, 2.6 (1.3-4.5) h in the multitest period and 3.7 (1.8-8.2) h in the reference period (p<0.01). Antibiotic therapy was initiated or adjusted in 68 patients based on early analysis results. The proportion of patients receiving appropriate antibiotic therapy within 48 h of blood sampling was higher in the multiplex PCR and multitest periods, (respectively 90% and 88%) than in the reference period (71%).
Conclusion: These results suggest rapid bacterial identification and antibiotic resistance tests are feasible, efficient and can expedite appropriate antibiotic therapy.
Keywords: MRSA/SA PCR; bacteremia; ePlex assay; oxidase test; β-Lacta test.
Copyright © 2023 Chatelard, Rousseau, Parmeland, Metral, Pariset and Vivier.
Conflict of interest statement
The GenMark ePlex system was provided free-of-charge to Saint Joseph Saint Luc hospital by the manufacturer.
Figures
References
-
- Banerjee R., Teng C. B., Cunningham S. A., Ihde S. M., Steckelberg J. M., Moriarty J. P., et al. (2015). Randomized trial of rapid multiplex polymerase chain reaction–based blood culture identification and susceptibility testing. Clin. Infect. Dis. 61 (7), 1071–1080. doi: 10.1093/cid/civ447 - DOI - PMC - PubMed
-
- Barlam T. F., Cosgrove S. E., Abbo L. M., MacDougall C., Schuetz A. N., Septimus E. J., et al. (2016). Executive summary: implementing an antibiotic stewardship program: guidelines by the infectious diseases society of America and the society for healthcare epidemiology of America. Clin. Infect. Dis. 62 (10), 1197–1202. doi: 10.1093/cid/ciw217 - DOI - PubMed
-
- Brown J., Paladino J. A. (2010). Impact of rapid methicillin-resistant staphylococcus aureus polymerase chain reaction testing on mortality and cost effectiveness in hospitalized patients with bacteraemia: a decision model. PharmacoEconomics. 28 (7), 567–575. doi: 10.2165/11533020-000000000-00000 - DOI - PubMed
-
- Bryant S., Almahmoud I., Pierre I., Bardet J., Touati S., Maubon D., et al. (2020). Evaluation of microbiological performance and the potential clinical impact of the ePlex® blood culture identification panels for the rapid diagnosis of bacteremia and fungemia. Front. Cell Infect. Microbiol. 10, 594951. doi: 10.3389/fcimb.2020.594951 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical