Galectin-1 induces a tumor-associated macrophage phenotype and upregulates indoleamine 2,3-dioxygenase-1
- PMID: 37534161
- PMCID: PMC10391608
- DOI: 10.1016/j.isci.2023.106984
Galectin-1 induces a tumor-associated macrophage phenotype and upregulates indoleamine 2,3-dioxygenase-1
Abstract
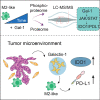
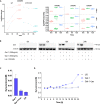
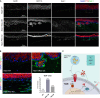
Galectins are a group of carbohydrate-binding proteins with a presumed immunomodulatory role and an elusive function on antigen-presenting cells. Here we analyzed the expression of galectin-1 and found upregulation of galectin-1 in the extracellular matrix across multiple tumors. Performing an in-depth and dynamic proteomic and phosphoproteomic analysis of human macrophages stimulated with galectin-1, we show that galectin-1 induces a tumor-associated macrophage phenotype with increased expression of key immune checkpoint protein programmed cell death 1 ligand 1 (PD-L1/CD274) and immunomodulator indoleamine 2,3-dioxygenase-1 (IDO1). Galectin-1 induced IDO1 and its active metabolite kynurenine in a dose-dependent manner through JAK/STAT signaling. In a 3D organotypic tissue model system equipped with genetically engineered tumorigenic epithelial cells, we analyzed the cellular source of galectin-1 in the extracellular matrix and found that galectin-1 is derived from epithelial and stromal cells. Our results highlight the potential of targeting galectin-1 in immunotherapeutic treatment of human cancers.
Keywords: Cancer; Immunology.
© 2023 The Authors.
Conflict of interest statement
Unrelated to the presented work, Hans Wandall owns stock and is a consultant for and co-founder of EbuMab, ApS. and GO-Therapeutics, Inc. HL is shareholder in Galecto Biotech AB, a company that is developing galectin inhibitors
Figures




References
-
- Liu F.-T., Rabinovich G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer. 2005;5:29–41. - PubMed
-
- Thijssen V.L., Heusschen R., Caers J., Griffioen A.W. Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim. Biophys. Acta. 2015;1855:235–247. - PubMed
-
- Johannes L., Jacob R., Leffler H. Galectins at a glance. J. Cell Sci. 2018;131:jcs208884. - PubMed
-
- Leffler H., Carlsson S., Hedlund M., Qian Y., Poirier F. Introduction to galectins. Glycoconj. J. 2002;19:433–440. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

