Promotion of skin wound healing using hypoimmunogenic epidermal cell sheets
- PMID: 37534237
- PMCID: PMC10393516
- DOI: 10.1016/j.reth.2023.07.003
Promotion of skin wound healing using hypoimmunogenic epidermal cell sheets
Abstract
Objective: The physiological process of wound healing is dynamic, continuous, and intricate. Nowadays, full-thickness burn wounds are treated by autologous skin transplantation. Unfortunately, when substantial burns develop, there are fewer donor sites accessible, making it difficult to satisfy the requirement for large-scale skin transplants and increasing the risk of patient mortality. This study investigated the possibility of using a newly created hypoimmunogenic epidermal cell sheet to heal skin wounds.
Methods: Transfection with lentivirus was used to generate Keratinocytes (KCs) that overexpress Indoleamine 2,3-Dioxygenase (IDO). Western blotting and quantitative polymerase chain reaction were used to measure IDO levels. To evaluate the function of IDO+ keratinocytes, CCK-8 and Transwell assays were performed. In cell sheet induction media, KCs and Fibroblasts (FBs) were cultured to yield epidermal cell sheets. The full-thickness skin excisions of BALB/c mice were transplanted with epidermal cell sheets. To assess the tumorigenicity of IDO+ keratinocytes, BALB/c nude mouse xenograft models were also used. CD3 and CD31 immunofluorescence labeling of wound tissue on day 12 to identify T lymphocyte infiltration and capillary development. ELISA measurement of IL-1 and TNF-α concentrations.
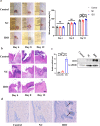
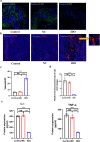
Results: IDO + keratinocytes dramatically enhanced the expression levels of IDO mRNA and protein, as well as the amount of kynurenine in the conditioned media of IDO+ keratinocytes, compared to the Control and NC groups. CD8+ T cell apoptosis was considerably greater in the IDO group than in the Control and NC groups. Nevertheless, the proliferation and migratory capabilities of IDO+ keratinocytes were not substantially different from those of the Control and NC groups. In vitro cultivation of the hypoimmunogenic epidermal cell sheet was effective. In vivo transplantation experiments demonstrated that IDO+ epidermal cell sheets can effectively promote wound healing without tumorigenicity, and IDO+ epidermal cell sheets may promote wound healing by decreasing the expression levels of inflammatory factors (TNF and IL-1) in wound tissue, decreasing CD3+ T lymphocytes, and increasing infiltration and new capillaries in wound tissue.
Conclusion: In this study, we successfully constructed the hypoimmunogenic epidermal cell sheet and demonstrated that the hypoimmunogenic epidermal cell sheet could accelerate wound healing.
Keywords: Allogenic skin substitute; Epidermal cell sheet; Indoleamine 2,3-dioxygenase; Skin; Wound healing.
© 2023 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures




Similar articles
-
[Clinical study of cell sheets containing allogeneic keratinocytes and fibroblasts for the treatment of partial-thickness burn wounds].Zhonghua Shao Shang Za Zhi. 2020 Mar 20;36(3):171-178. doi: 10.3760/cma.j.cn501120-20191113-00426. Zhonghua Shao Shang Za Zhi. 2020. PMID: 32241042 Chinese.
-
[Effects and mechanism of rat epidermal stem cells treated with exogenous vascular endothelial growth factor on healing of deep partial-thickness burn wounds in rats].Zhonghua Shao Shang Za Zhi. 2020 Mar 20;36(3):195-203. doi: 10.3760/cma.j.cn501120-20191125-00441. Zhonghua Shao Shang Za Zhi. 2020. PMID: 32241045 Chinese.
-
Using paracrine effects of Ad-MSCs on keratinocyte cultivation and fabrication of epidermal sheets for improving clinical applications.Cell Tissue Bank. 2018 Dec;19(4):531-547. doi: 10.1007/s10561-018-9702-5. Epub 2018 Aug 13. Cell Tissue Bank. 2018. PMID: 30105667
-
Immunoprotective role of indoleamine 2,3-dioxygenase in engraftment of allogenic skin substitute in wound healing.J Burn Care Res. 2012 May-Jun;33(3):364-70. doi: 10.1097/BCR.0b013e318235836e. J Burn Care Res. 2012. PMID: 22249100 Review.
-
Emerging Technologies in Scar Management: The Role of Allogeneic Cells.2020 Dec 8. In: Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, editors. Textbook on Scar Management: State of the Art Management and Emerging Technologies [Internet]. Cham (CH): Springer; 2020. Chapter 51. 2020 Dec 8. In: Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, editors. Textbook on Scar Management: State of the Art Management and Emerging Technologies [Internet]. Cham (CH): Springer; 2020. Chapter 51. PMID: 36351100 Free Books & Documents. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

