Bevacizumab in recurrent WHO grades II-III glioma
- PMID: 37534252
- PMCID: PMC10391542
- DOI: 10.3389/fonc.2023.1212714
Bevacizumab in recurrent WHO grades II-III glioma
Abstract
Purpose: The management of recurrent WHO grades II-III (rGII-III) glioma is not well established. This study describes the clinical outcomes in patients who received bevacizumab as rescue treatment.
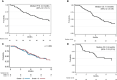
Methods: In this retrospective study, the main inclusion criteria were as follows: adult patients with histologicaly proved rGII-III glioma according 2016 WHO classification treated with bevacizumab from 2011 to 2019, T1 contrast enhancement on MRI. Efficacy was assessed using the high-grade glioma 2017 Response Assessment in Neuro-Oncology criteria. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method.
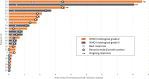
Results: Eighty-one patients were included (M/F ratio: 1.7, median age at diagnosis: 38 years) among whom 46 (56.8%) had an initial diagnosis of grade II glioma. Previous treatments included at least one surgical intervention, radiotherapy (98.8%), and ≥ 2 chemotherapy lines (64.2%). After bevacizumab initiation, partial response, stable disease, and progressive disease were observed in 27.2%, 22.2%, and 50.6% of patients. The median PFS and OS were 4.9 months (95% confidence interval [CI] 3.7-6.1) and 7.6 months (95% CI 5.5-9.9). Bevacizumab severe toxicity occurred in 12.3%. Twenty-four (29.6%) patients discontinued bevacizumab without radiological progression. Oligodendroglioma and age ≥ 38 years at diagnosis were more frequent in this subgroup (odds ratio = 0.24, 95% CI 0.07-0.84, p = 0.023 and 0.36, 95% CI 0.13-0.99, p = 0.042). Ten of these 24 patients were alive at 12 months and two patients at 8 years after bevacizumab initiation, without any subsequent treatment.
Conclusion: Bevacizumab can be an option for heavily pretreated patients with rGII-III glioma with contrast enhancement. In our study, bevacizumab displayed prolonged activity in a subgroup of patients.
Keywords: anaplastic glioma; astrocytoma; bevacizumab; oligodendroglioma; recurrent glioma; transformed low grade glioma.
Copyright © 2023 Annakib, Rigau, Darlix, Gozé, Duffau, Bauchet, Jarlier and Fabbro.
Conflict of interest statement
HD is an Integra speaker’s bureau member. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

