Sex differences in pituitary corticotroph excitability
- PMID: 37534368
- PMCID: PMC10391550
- DOI: 10.3389/fphys.2023.1205162
Sex differences in pituitary corticotroph excitability
Abstract
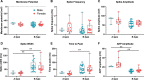
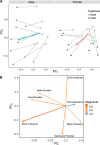
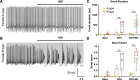
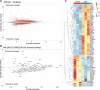
Stress-related illness represents a major burden on health and society. Sex differences in stress-related disorders are well documented, with women having twice the lifetime rate of depression compared to men and most anxiety disorders. Anterior pituitary corticotrophs are central components of the hypothalamic-pituitary-adrenal (HPA) axis, receiving input from hypothalamic neuropeptides corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP), while regulating glucocorticoid output from the adrenal cortex. The dynamic control of electrical excitability by CRH/AVP and glucocorticoids is critical for corticotroph function; however, whether corticotrophs contribute to sexually differential responses of the HPA axis, which might underlie differences in stress-related disorders, is very poorly understood. Using perforated patch clamp electrophysiology in corticotrophs from mice expressing green fluorescent protein under the control of the Pomc promoter, we characterized basal and secretagogue-evoked excitability. Both male and female corticotrophs show predominantly single-spike action potentials under basal conditions; however, males predominantly display spikes with small-amplitude (<20 mV) afterhyperpolarizations (B-type), whereas females displayed a mixture of B-type spikes and spikes with a large-amplitude (>25 mV) afterhyperpolarization (A-type). In response to CRH, or CRH/AVP, male cells almost exclusively transition to a predominantly pseudo-plateau bursting, whereas only female B-type cells display bursting in response to CRH±AVP. Treatment of male or female corticotrophs with 1 nM estradiol (E2) for 24-72 h has no effect on the proportion of cells with A- or B-type spikes in either sex. However, E2 results in the cessation of CRH-induced bursting in both male and female corticotrophs, which can be partially reversed by adding a BK current using a dynamic clamp. RNA-seq analysis of purified corticotrophs reveals extensive differential gene expression at the transcriptional level, including more than 71 mRNAs encoding ion channel subunits. Interestingly, there is a two-fold lower level (p < 0.01) of BK channel pore-forming subunit (Kcnma1) expression in females compared to males, which may partially explain the decrease in CRH-induced bursting. This study identified sex differences at the level of the anterior pituitary corticotroph ion channel landscape and control of both spontaneous and CRH-evoked excitability. Determining the mechanisms of sex differences of corticotroph and HPA activity at the cellular level could be an important step for better understanding, diagnosing, and treating stress-related disorders.
Keywords: BK channel; HPA axis; RNA-seq; corticotroph; electrophysiology; sex differences.
Copyright © 2023 Duncan, Romanò, Nair, Murray, Le Tissier and Shipston.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

