Use of canakinumab and remdesivir in moderate-severe COVID-19 patients: A retrospective analysis
- PMID: 37534444
- PMCID: PMC10402280
- DOI: 10.1177/03946320231189993
Use of canakinumab and remdesivir in moderate-severe COVID-19 patients: A retrospective analysis
Abstract
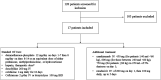
Objectives: The dysregulated immune response occurring upon COVID-19 infection can lead to tissue damage and organ failure. Different therapeutic strategies are needed to cope with the current and future outspread of COVID-19, including antiviral and anti-inflammatory agents. We describe the outcome of hospitalized patients treated with canakinumab and remdesivir plus the standard of care therapy. Methods: This observational study describes the outcome of the combination of canakinumab (450 mg for patients ≥40 and <60 kg, 600 mg for those ≥60 and <80 kg, or 750 mg for patients ≥80 kg) and 200 mg remdesivir intravenous infusion, plus standard of care (SOC), in 17 moderate-to-severe COVID-19 patients hospitalized in the "Annunziata" Hospital, Cosenza, Italy, between August and November 2021. Hematological markers, biochemical, and hemogasanalysis values at baseline versus day 7 of combination treatment were compared by paired t test after checking for normal distribution and correcting for multiple comparison. Results: The median age of patients was 64 years (range: 39-85), and the median hospitalization time (calculated on the 16 patients that were not transferred to intensive care unit) was of 12.5 days (range: 7-35 days); 15/17 patients (88%) did not experience complications. After 7 days of combination therapy, all the inflammatory parameters were significantly reduced with the exception of procalcitonin; moreover, hematological prognostic markers such neutrophil-to-lymphocyte ratio, CRP-to-lymphocyte ratio, and derived neutrophil-to-lymphocyte ratio reduced. Overall, 16/17 patients (94%) recovered after 14 days. Conclusions: Canakinumab and remdesivir treatment, in addition to SOC, in the early stage of moderate-to-severe COVID-19 showed promising outcomes in terms of safety and effectiveness potentially leading to a reduction in inflammatory and hematological prognostic markers after 7 days of treatment.
Keywords: COVID-19; canakinumab; hematological markers; inflammatory markers; remdesivir.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- WHO (2023) WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (accessed 23 January 2023).
-
- WHO (2023) Tracking SARS-CoV-2 Variants. https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed 23 January 2023).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous