Cardiac and Metabolic Effects of Dapagliflozin in Heart Failure With Preserved Ejection Fraction: The CAMEO-DAPA Trial
- PMID: 37534453
- PMCID: PMC10529848
- DOI: 10.1161/CIRCULATIONAHA.123.065134
Cardiac and Metabolic Effects of Dapagliflozin in Heart Failure With Preserved Ejection Fraction: The CAMEO-DAPA Trial
Abstract
Background: Sodium-glucose cotransporter-2 inhibitors reduce risk of hospitalization for heart failure in patients who have heart failure with preserved ejection fraction (HFpEF), but the hemodynamic mechanisms underlying these benefits remain unclear. This study sought to determine whether treatment with dapagliflozin affects pulmonary capillary wedge pressure (PCWP) at rest and during exercise in patients with HFpEF.
Methods: This was a single-center, double-blinded, randomized, placebo-controlled trial testing the effects of 10 mg of dapagliflozin once daily in patients with HFpEF. Patients with New York Heart Association class II or III heart failure, ejection fraction ≥50%, and elevated PCWP during exercise were recruited. Cardiac hemodynamics were measured at rest and during exercise using high-fidelity micromanometers at baseline and after 24 weeks of treatment. The primary end point was a change from baseline in rest and peak exercise PCWPs that incorporated both measurements, and was compared using a mixed-model likelihood ratio test. Key secondary end points included body weight and directly measured blood and plasma volumes. Expired gas analysis was performed evaluate oxygen transport in tandem with arterial lactate sampling.
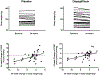
Results: Among 38 patients completing baseline assessments (median age 68 years; 66% women; 71% obese), 37 completed the trial. Treatment with dapagliflozin resulted in reduction in the primary end point of change in PCWP at rest and during exercise at 24 weeks relative to treatment with placebo (likelihood ratio test for overall changes in PCWP; P<0.001), with lower PCWP at rest (estimated treatment difference [ETD], -3.5 mm Hg [95% CI, -6.6 to -0.4]; P=0.029) and maximal exercise (ETD, -5.7 mm Hg [95% CI, -10.8 to -0.7]; P=0.027). Body weight was reduced with dapagliflozin (ETD, -3.5 kg [95% CI, -5.9 to -1.1]; P=0.006), as was plasma volume (ETD, -285 mL [95% CI, -510 to -60]; P=0.014), but there was no significant effect on red blood cell volume. There were no differences in oxygen consumption at 20-W or peak exercise, but dapagliflozin decreased arterial lactate at 20 W (-0.70 ± 0.77 versus 0.37 ± 1.29 mM; P=0.006).
Conclusions: In patients with HFpEF, treatment with dapagliflozin reduces resting and exercise PCWP, along with the favorable effects on plasma volume and body weight. These findings provide new insight into the hemodynamic mechanisms of benefit with sodium-glucose cotransporter-2 inhibitors in HFpEF.
Registration: URL: https://www.
Clinicaltrials: gov; Unique identifier: NCT04730947.
Keywords: diastolic pressure; exercise; heart failure.
Conflict of interest statement
Figures


References
-
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Pina IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M and Investigators EM-PT. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021.
-
- Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O’Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderang U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM, Committees DT and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022.
-
- Cowie MR and Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17:761–772. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

