Nitridochromate(IV): LiSr2[CrN3]
- PMID: 37534772
- PMCID: PMC10428211
- DOI: 10.1021/acs.inorgchem.3c01697
Nitridochromate(IV): LiSr2[CrN3]
Abstract
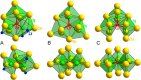
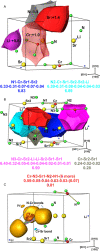
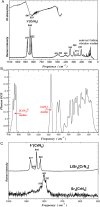
The quaternary nitridochromate(IV) LiSr2[CrN3] crystallizes in a new structure type with the non-centrosymmetric space group P21 (no. 4) with a = 5.5685(7) Å, b = 5.3828(8) Å, c = 7.5381(1) Å, and β = 92.291(8)°. Predominant structural features of the compound are slightly nonplanar trigonal units [CrN3]5-, which are connected by three-fold coordinated lithium to form slabs in the (001) plane. Shorter Cr-N bond lengths in comparison with reported nitridochromates(III), as well as diamagnetic behavior and vibrational spectroscopy data indicate Cr(IV), which is in a good agreement with the charge balance. According to electronic structure calculations, the compound is a semiconductor with a band gap of 1.19 eV.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Höhn P.; Niewa R.. Nitrides of Non-main Group Elements. In Handbook of Solid State Chemistry; Wiley VCH, 2017; Vol 1.
-
- Blix R. Röntgenanalyse des Chrom—Stickstoffsystems nebst einer orientierenden Konstitutionsuntersuchung des stickstoffhaltigen Ferrochroms. Z. Phys. Chem. 1929, 3B, 229–239. 10.1515/zpch-1929-0317. - DOI
-
- Bailey M. S.; DiSalvo F. J. Cr(v)–Cr(v) dimers in Ae4[Cr2N6](Ae = Ca and Sr). Dalton Trans. 2003, 2621–2625. 10.1039/b302015k. - DOI
-
- Tennstedt A.; Kniep R.; Hüber M.; Haase W. Ba5[CrN4]N: Das erste Nitridochromat(V). Z. Anorg. Allg. Chem. 1995, 621, 511–515. 10.1002/zaac.19956210402. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

