Selective targeting of lectins and their macropinocytosis in urothelial tumours: translation from in vitro to ex vivo
- PMID: 37535087
- PMCID: PMC10624759
- DOI: 10.1007/s00418-023-02224-2
Selective targeting of lectins and their macropinocytosis in urothelial tumours: translation from in vitro to ex vivo
Abstract
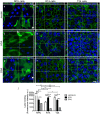
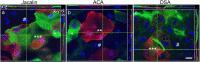
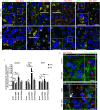
Urinary bladder cancer can be treated by intravesical application of therapeutic agents, but the specific targeting of cancer urothelial cells and the endocytotic pathways of the agents are not known. During carcinogenesis, the superficial urothelial cells exhibit changes in sugar residues on the apical plasma membranes. This can be exploited for selective targeting from the luminal side of the bladder. Here we show that the plant lectins Jacalin (from Artocarpus integrifolia), ACA (from Amaranthus caudatus) and DSA (from Datura stramonium) selectively bind to the apical plasma membrane of low- (RT4) and high-grade (T24) cancer urothelial cells in vitro and urothelial tumours ex vivo. The amount of lectin binding was significantly different between RT4 and T24 cells. Endocytosis of lectins was observed only in cancer urothelial cells and not in normal urothelial cells. Transmission electron microscopy analysis showed macropinosomes, endosome-like vesicles and multivesicular bodies filled with lectins in RT4 and T24 cells and also in cells of urothelial tumours ex vivo. Endocytosis of Jacalin and ACA in cancer cells was decreased in vitro after addition of inhibitor of macropinocytosis 5-(N-ethyl-N-isopropyl) amiloride (EIPA) and increased after stimulation of macropinocytosis with epidermal growth factor (EGF). Clathrin, caveolin and flotillin did not colocalise with lectins. These results confirm that the predominant mechanism of lectin endocytosis in cancer urothelial cells is macropinocytosis. Therefore, we propose that lectins in combination with conjugated therapeutic agents are promising tools for improved intravesical therapy by targeting cancer cells.
Keywords: Cancer urothelial cells; Endocytosis; Glycosylation; Lectins; Macropinocytosis; Urinary bladder tumours.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

