Use and Cost of Sustained-Release Corticosteroids for Cataract Surgery Under the Medicare Pass-Through Program
- PMID: 37535374
- PMCID: PMC10401390
- DOI: 10.1001/jamaophthalmol.2023.3389
Use and Cost of Sustained-Release Corticosteroids for Cataract Surgery Under the Medicare Pass-Through Program
Abstract
Importance: Sustained-release corticosteroids offer the potential of improved compliance and greater patient convenience for anti-inflammatory treatment after cataract surgery. However, they are substantially more expensive than postoperative corticosteroid eye drops, which have historically been standard care.
Objective: To examine the use and cost of sustained-release corticosteroids in patients with Medicare who underwent cataract surgery in the US during the temporary pass-through reimbursement program period.
Design, setting, and participants: This cross-sectional study examined Medicare fee-for-service (FFS) claims from beneficiaries with at least 12 continuous months of Medicare enrollment who underwent at least 1 cataract surgery from March 2019 through December 2021. Patients younger than 65 years, those with missing demographic information, those who had more than 1 cataract surgery on each eye, and those who received more than 1 corticosteroid on the day of surgery were excluded. Cataract surgeries with concurrent use of dexamethasone intraocular suspension 9% or dexamethasone ophthalmic insert were identified. Information on surgeon demographic characteristics and costs of surgery and drugs were extracted. Data were analyzed from June 15 to December 4, 2022.
Exposure: Use of dexamethasone intraocular suspension 9% or dexamethasone ophthalmic insert during cataract surgery.
Main outcome measures: Utilization rate and cost of dexamethasone intraocular suspension 9% and dexamethasone ophthalmic insert among Medicare FFS beneficiaries who underwent cataract surgery.
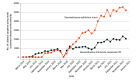
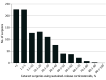
Results: A total of 4 252 532 cataract surgeries in Medicare FFS beneficiaries (mean [SD] age, 74.8 [5.8] years; 1 730 811 male [40.7%] and 2 521 721 female [59.3%]) were performed by 12 284 ophthalmologists (8876 male [72.3%], 2877 female [23.4%], and 531 sex unknown [4.3%]). In all, 34 627 beneficiaries (0.8%) received dexamethasone intraocular suspension 9% and 73 430 (1.7%) received a dexamethasone ophthalmic insert; the use of both drugs increased over the study period. The mean (SD) Medicare allowed charges for dexamethasone intraocular suspension 9% and dexamethasone ophthalmic insert were $531.47 ($141.52) and $538.49 ($63.79), respectively.
Conclusions and relevance: Despite offering the potential of improved compliance and greater patient convenience, findings of this study suggest that sustained-release corticosteroid use during cataract surgery was low and associated with cost increases to the health care system vs conventional postoperative eye drops. As these new products must be priced high enough to qualify for the Medicare pass-through program, unreasonable cost may have been a deterrent to their use, suggesting that the current Medicare reimbursement rules may not be appropriate for sustained-release postoperative corticosteroids in cataract surgery.
Conflict of interest statement
Figures
Comment in
-
Pass-Through Payments, Cost, and Convenience in Hospital Outpatient Departments and Ambulatory Surgical Centers.JAMA Ophthalmol. 2023 Sep 1;141(9):851-852. doi: 10.1001/jamaophthalmol.2023.3424. JAMA Ophthalmol. 2023. PMID: 37535383 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

