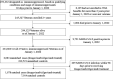
Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: retrospective analysis of National Veterans Health Administration electronic data
- PMID: 37535398
- PMCID: PMC10470809
- DOI: 10.1128/mbio.01024-23
Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: retrospective analysis of National Veterans Health Administration electronic data
Abstract
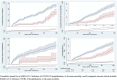
Little is known regarding the effectiveness of tixagevimab/cilgavimab in preventing SARS-CoV-2 infection in vaccinated immunocompromised patients, particularly after the emergence of the Omicron variant. In this retrospective cohort study with exact matching and propensity score adjustment within the U.S. Department of Veterans Affairs (VA) healthcare system, we selected immunocompromised veterans age ≥18 years as of 1 January 2022, receiving VA healthcare. We compared a cohort of 1,878 patients treated with at least one dose of intramuscular tixagevimab/cilgavimab to 7,014 matched controls selected from patients who met study criteria but were not treated. Patients were followed through 15 June 2022, or until death, whichever occurred earlier. The primary outcome was a composite of SARS-CoV-2 infection, COVID-19-related hospitalization, and all-cause mortality. We used Cox proportional hazards modeling to estimate the hazard ratios (HRs) and 95% CI for the association between receipt of tixagevimab/cilgavimab and outcomes. Most (73%) tixagevimab/cilgavimab recipients were ≥65 years old, and 80% had ≥3 mRNA vaccine doses or two doses of Ad26.COV2. Compared to matched controls, recipients had a lower incidence of the composite COVID-19 outcome (49/1,878 [2.6%] versus 312/7,014 [4.4%]; HR 0.35; 95% CI, 0.24-0.52), and individually SARS-CoV-2 infection (HR 0.44; 95% CI, 0.22-0.88), COVID-19 hospitalization (HR 0.24; 95% CI, 0.10-0.59), and all-cause mortality (HR 0.32; 95% CI, 0.19-0.55). In conclusion, tixagevimab/cilgavimab was associated with lower rates of SARS-CoV-2 infection and severe COVID-19 during the Omicron BA.1, BA.2, and BA.2.12.1 surge. IMPORTANCE SARS-CoV-2 remains an ongoing global health crisis that justifies continued efforts to validate and expand, when possible, knowledge on the efficacy of available vaccines and treatments. Clinical trials have been limited due to fast tracking of medications for mitigation of the COVID-19 pandemic for the general population. We present a real-world analysis, using electronic health record data, of the effectiveness of tixagevimab/cilgavimab for the prevention of COVID-19 infection in the unique population of U.S. veterans. Unlike those in the PROVENT clinical trial from which the emergency use authorization for tixagevimab/cilgavimab as a preventative treatment arose, the veterans population is highly immunocompromised and nearly 96% totally vaccinated. These demographics allowed us to analyze the effectiveness of tixagevimab/cilgavimab in preventing COVID-19 under different conditions in a more fragile population than that of the initial clinical trial.
Keywords: COVID-19; SARS-CoV-2; monoclonal antibodies; prevention; propensity score matching; real-world data.
Conflict of interest statement
V.C.M. has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences and ViiV. Y.Y.-X., G.Z., C.K., and J.S. reported receiving grants from the U.S. Food and Drug Administration through an interagency agreement with the Veterans Health Administration and from the U.S. Department of Veterans Affairs Office of Rural Health. Y.Y.-X., G.Z., C.K., and J.S. also reported receiving funding from Pfizer to the U.S. Department of Veterans Affairs for other research projects outside the submitted work. R.A.B. is supported by VA/BLRD VA SHIELD (821-SD-null-41942); Y.Y.-X., V.C.M., and R.A.B. are supported by VA/BLRD VA SEQCURE (821-SD-ID-42403). A.A.G. received COVID-19 research project funding from the National Institutes of Health, Department of Defense, Centers for Disease Control and Prevention, AbbVie, and Faron Pharmaceuticals, outside the submitted work.
Figures
References
-
- Embi PJ, Levy ME, Naleway AL, Patel P, Gaglani M, Natarajan K, Dascomb K, Ong TC, Klein NP, Liao I-C, Grannis SJ, Han J, Stenehjem E, Dunne MM, Lewis N, Irving SA, Rao S, McEvoy C, Bozio CH, Murthy K, Dixon BE, Grisel N, Yang D-H, Goddard K, Kharbanda AB, Reynolds S, Raiyani C, Fadel WF, Arndorfer J, Rowley EA, Fireman B, Ferdinands J, Valvi NR, Ball SW, Zerbo O, Griggs EP, Mitchell PK, Porter RM, Kiduko SA, Blanton L, Zhuang Y, Steffens A, Reese SE, Olson N, Williams J, Dickerson M, McMorrow M, Schrag SJ, Verani JR, Fry AM, Azziz-Baumgartner E, Barron MA, Thompson MG, DeSilva MB. 2021. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults - nine states, January-September 2021. MMWR Morb Mortal Wkly Rep 70:1553–1559. doi:10.15585/mmwr.mm7044e3 - DOI - PMC - PubMed
-
- Kennedy NA, Lin S, Goodhand JR, Chanchlani N, Hamilton B, Bewshea C, Nice R, Chee D, Cummings JF, Fraser A, Irving PM, Kamperidis N, Kok KB, Lamb CA, Macdonald J, Mehta S, Pollok RC, Raine T, Smith PJ, Verma AM, Jochum S, McDonald TJ, Sebastian S, Lees CW, Powell N, Ahmad T, Contributors to the CLARITY IBD study . 2021. Infliximab is associated with attenuated Immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 70:1884–1893. doi:10.1136/gutjnl-2021-324789 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous