Repeated seizures lead to progressive ventilatory dysfunction in SS Kcnj16-/- rats
- PMID: 37535709
- PMCID: PMC10642517
- DOI: 10.1152/japplphysiol.00072.2023
Repeated seizures lead to progressive ventilatory dysfunction in SS Kcnj16-/- rats
Abstract
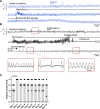
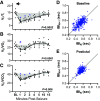
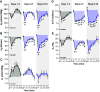
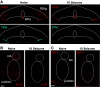
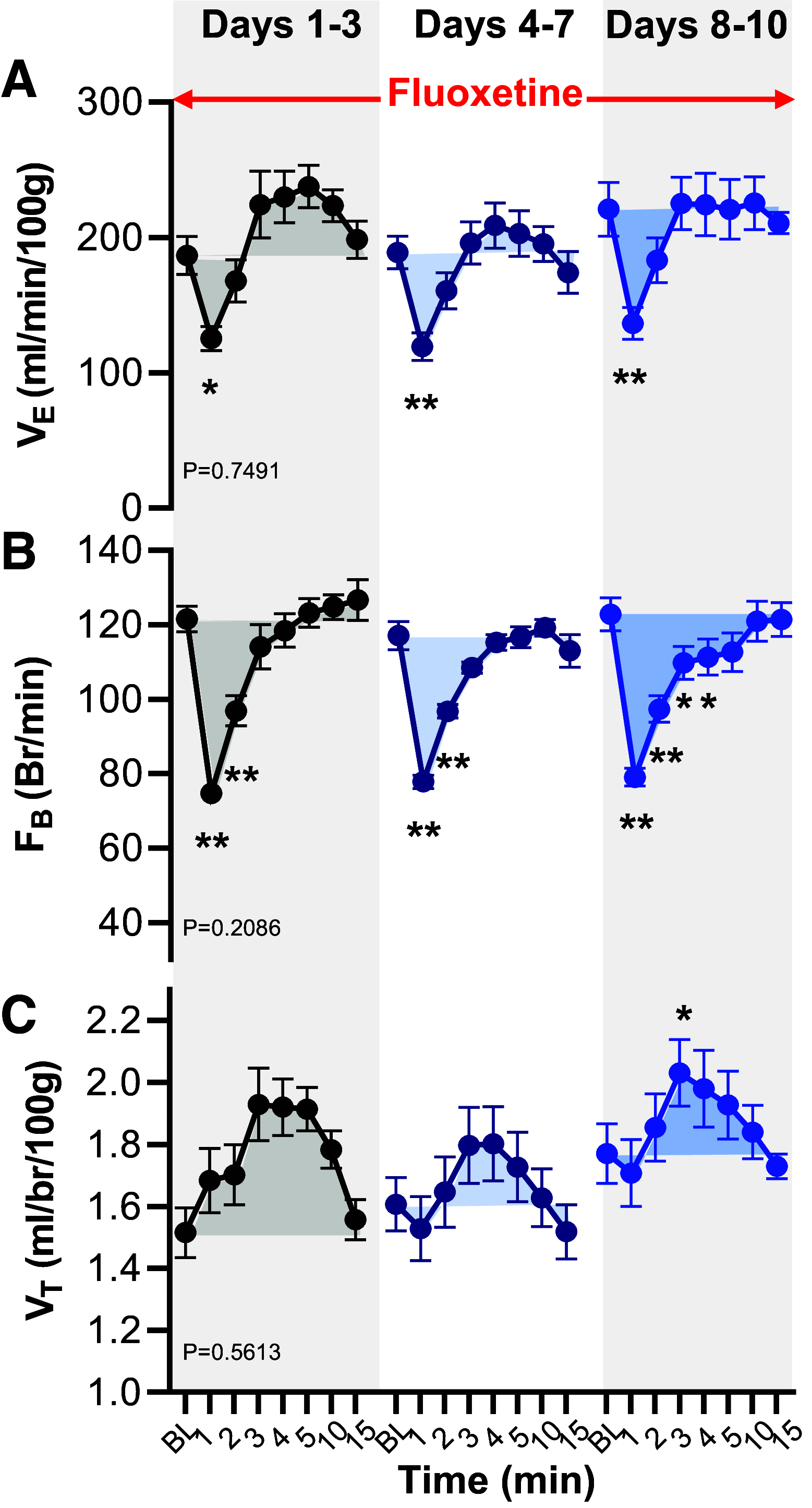
Patients with uncontrolled epilepsy experience repeated seizures putting them at increased risk for sudden unexpected death in epilepsy (SUDEP). Data from human patients have led to the hypothesis that SUDEP results from severe cardiorespiratory suppression after a seizure, which may involve pathological deficiencies in the brainstem serotonin (5-HT) system. Rats with a genomic Kcnj16 mutation (SSKcnj16-/- rats) are susceptible to sound-induced generalized tonic-clonic seizures (GTCS) which, when repeated once daily for up to 10 days (10-day seizure protocol), increased mortality, particularly in male rats. Here, we test the hypothesis that repeated seizures across the 10-day protocol will cause a progressive ventilatory dysfunction due to time-dependent 5-HT deficiency. Initial severe seizures led to ictal and postictal apneas and transient decreases in breathing frequency, ventilatory drive, breath-to-breath variability, and brief hypoventilation. These seizure-induced effects on ventilation were exacerbated with increasing seizures and ventilatory chemoreflexes became further impaired after repeated seizures. Tissue analyses of key brainstem regions controlling breathing showed time-dependent 5-HT system suppression and increased immunoreactivity for IBA-1 (microglial marker) without changes in overall cell counts at 3, 7, and 10 days of seizures. Fluoxetine treatment in SSKcnj16-/- rats prevented repeated seizure-induced progressive respiratory suppression but failed to prevent seizure-related mortality. We conclude that repeated seizures cause a progressive compromise of ventilatory control in the immediate postictal period largely mediated by serotonin system suppression in brainstem regions of respiratory control. However, other unknown factors contribute to overall survival following repeated seizures in this model.NEW & NOTEWORTHY This study demonstrated that repeated seizures in a novel rat model (SSKcnj16-/- rats) caused a progressively greater ventilatory dysfunction in the immediate postictal period associated with brainstem serotonin (5-HT) suppression. Augmenting brain 5-HT with a selective serotonin reuptake inhibitor prevented the progressive ventilatory dysfunction induced by repeated seizures but failed to prevent seizure-related mortality, suggesting that repeated seizures may lead to cardiorespiratory suppression and failure through multiple mechanisms.
Keywords: SUDEP; breathing variability; respiratory depression; seizures; serotonin.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1545–1602, 2016. [Erratum in Lancet 389: e1, 2017]. doi:10.1016/S0140-6736(16)31678-6. - DOI - PMC - PubMed
-
- Harden C, Tomson T, Gloss D, Buchhalter J, Cross JH, Donner E, French JA, Gil-Nagel A, Hesdorffer DC, Smithson WH, Spitz MC, Walczak TS, Sander JW, Ryvlin P. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 88: 1674–1680, 2017. [Erratum in Neurology 93: 982, 2019]. doi:10.1212/WNL.0000000000003685. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

