The Experimentalist's Guide to Machine Learning for Small Molecule Design
- PMID: 37535819
- PMCID: PMC10880109
- DOI: 10.1021/acsabm.3c00054
The Experimentalist's Guide to Machine Learning for Small Molecule Design
Abstract
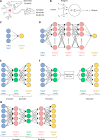
Initially part of the field of artificial intelligence, machine learning (ML) has become a booming research area since branching out into its own field in the 1990s. After three decades of refinement, ML algorithms have accelerated scientific developments across a variety of research topics. The field of small molecule design is no exception, and an increasing number of researchers are applying ML techniques in their pursuit of discovering, generating, and optimizing small molecule compounds. The goal of this review is to provide simple, yet descriptive, explanations of some of the most commonly utilized ML algorithms in the field of small molecule design along with those that are highly applicable to an experimentally focused audience. The algorithms discussed here span across three ML paradigms: supervised learning, unsupervised learning, and ensemble methods. Examples from the published literature will be provided for each algorithm. Some common pitfalls of applying ML to biological and chemical data sets will also be explained, alongside a brief summary of a few more advanced paradigms, including reinforcement learning and semi-supervised learning.
Keywords: QSAR; data analysis; drug design; experimentalist friendly; machine learning; small molecule design.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Sundstrom M.; Pelander A.; Angerer V.; Hutter M.; Kneisel S.; Ojanpera I. A high-sensitivity ultra-high performance liquid chromatography/high-resolution time-of-flight mass spectrometry (UHPLC-HR-TOFMS) method for screening synthetic cannabinoids and other drugs of abuse in urine. Anal Bioanal Chem. 2013, 405 (26), 8463–8474. 10.1007/s00216-013-7272-8. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
