Observing Extracellular Vesicles Originating from Endothelial Cells in Vivo Demonstrates Improved Astrocyte Function Following Ischemic Stroke via Aggregation-Induced Emission Luminogens
- PMID: 37535897
- PMCID: PMC10448755
- DOI: 10.1021/acsnano.3c05309
Observing Extracellular Vesicles Originating from Endothelial Cells in Vivo Demonstrates Improved Astrocyte Function Following Ischemic Stroke via Aggregation-Induced Emission Luminogens
Abstract
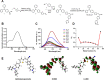
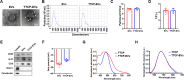
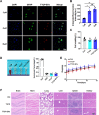
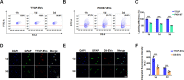
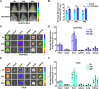
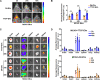
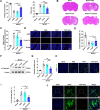
Extracellular vesicles (EVs) obtained from endothelial cells (ECs) have significant therapeutic potential in the clinical management of individuals with ischemic stroke (IS) because they effectively treat ischemic stroke in animal models. However, because molecular probes with both high labeling efficiency and tracer stability are lacking, monitoring the actions of EC-EVs in the brain remains difficult. The specific intracellular targets in the brain that EC-EVs act on to produce their protective effects are still unknown, greatly impeding their use in clinical settings. For this research, we created a probe that possessed aggregation-induced emission (AIE) traits (namely, TTCP), enabling the effective labeling of EC-EVs while preserving their physiological properties. In vitro, TTCP simultaneously had a higher EC-EV labeling efficiency and better tracer stability than the commercial EV tags PKH-67 and DiI. In vivo, TTCP precisely tracked the actions of EC-EVs in a mouse IS model without influencing their protective effects. Furthermore, through the utilization of TTCP, it was determined that astrocytes were the specific cells affected by EC-EVs and that EC-EVs exhibited a safeguarding impact on astrocytes following cerebral ischemia-reperfusion (I/R) injury. These protective effects encompassed the reduction of the inflammatory reaction and apoptosis as well as the enhancement of cell proliferation. Further analysis showed that miRNA-155-5p carried by EC-EVs is responsible for these protective effects via regulation of the c-Fos/AP-1 pathway; this information provided a strategy for IS therapy. In conclusion, TTCP has a high EC-EV labeling efficiency and favorable in vivo tracer stability during IS therapy. Moreover, EC-EVs are absorbed by astrocytes during cerebral I/R injury and promote the restoration of neurological function through the regulation of the c-Fos/AP-1 signaling pathway.
Keywords: aggregation-induced emission; astrocytes; extracellular vesicles; fluorescence imaging; human umbilical vein endothelial cells; ischemic stroke.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 301 Acute and Chronic Diseases and Injuries in 188 Countries, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2015, 386 (9995), 743–800. 10.1016/S0140-6736(15)60692-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

