Chemoenzymatic Syntheses of Fluorine-18-Labeled Disaccharides from [18F] FDG Yield Potent Sensors of Living Bacteria In Vivo
- PMID: 37535945
- PMCID: PMC10436271
- DOI: 10.1021/jacs.3c03338
Chemoenzymatic Syntheses of Fluorine-18-Labeled Disaccharides from [18F] FDG Yield Potent Sensors of Living Bacteria In Vivo
Abstract
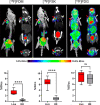
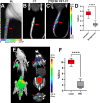
Chemoenzymatic techniques have been applied extensively to pharmaceutical development, most effectively when routine synthetic methods fail. The regioselective and stereoselective construction of structurally complex glycans is an elegant application of this approach that is seldom applied to positron emission tomography (PET) tracers. We sought a method to dimerize 2-deoxy-[18F]-fluoro-d-glucose ([18F]FDG), the most common tracer used in clinical imaging, to form [18F]-labeled disaccharides for detecting microorganisms in vivo based on their bacteria-specific glycan incorporation. When [18F]FDG was reacted with β-d-glucose-1-phosphate in the presence of maltose phosphorylase, the α-1,4- and α-1,3-linked products 2-deoxy-[18F]-fluoro-maltose ([18F]FDM) and 2-deoxy-2-[18F]-fluoro-sakebiose ([18F]FSK) were obtained. This method was further extended with the use of trehalose (α,α-1,1), laminaribiose (β-1,3), and cellobiose (β-1,4) phosphorylases to synthesize 2-deoxy-2-[18F]fluoro-trehalose ([18F]FDT), 2-deoxy-2-[18F]fluoro-laminaribiose ([18F]FDL), and 2-deoxy-2-[18F]fluoro-cellobiose ([18F]FDC). We subsequently tested [18F]FDM and [18F]FSK in vitro, showing accumulation by several clinically relevant pathogens including Staphylococcus aureus and Acinetobacter baumannii, and demonstrated their specific uptake in vivo. Both [18F]FDM and [18F]FSK were stable in human serum with high accumulation in preclinical infection models. The synthetic ease and high sensitivity of [18F]FDM and [18F]FSK to S. aureus including methicillin-resistant (MRSA) strains strongly justify clinical translation of these tracers to infected patients. Furthermore, this work suggests that chemoenzymatic radiosyntheses of complex [18F]FDG-derived oligomers will afford a wide array of PET radiotracers for infectious and oncologic applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Update of
-
Chemoenzymatic syntheses of fluorine-18-labeled disaccharides from [ 18 F]FDG yield potent sensors of living bacteria in vivo.bioRxiv [Preprint]. 2023 May 20:2023.05.20.541529. doi: 10.1101/2023.05.20.541529. bioRxiv. 2023. Update in: J Am Chem Soc. 2023 Aug 16;145(32):17632-17642. doi: 10.1021/jacs.3c03338. PMID: 37293043 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

