Tryptophan metabolism promotes immune evasion in human pancreatic β cells
- PMID: 37536063
- PMCID: PMC10412781
- DOI: 10.1016/j.ebiom.2023.104740
Tryptophan metabolism promotes immune evasion in human pancreatic β cells
Abstract
Background: To resist the autoimmune attack characteristic of type 1 diabetes, insulin producing pancreatic β cells need to evade T-cell recognition. Such escape mechanisms may be conferred by low HLA class I (HLA-I) expression and upregulation of immune inhibitory molecules such as Programmed cell Death Ligand 1 (PD-L1).
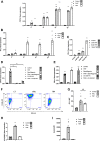
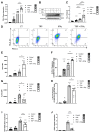
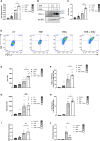
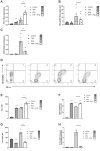
Methods: The expression of PD-L1, HLA-I and CXCL10 was evaluated in the human β cell line, ECN90, and in primary human and mouse pancreatic islets. Most genes were determined by real-time RT-PCR, flow cytometry and Western blot. Activator and inhibitor of the AKT signaling were used to modulate PD-L1 induction. Key results were validated by monitoring activity of CD8+ Jurkat T cells presenting β cell specific T-cell receptor and transduced with reporter genes in contact culture with the human β cell line, ECN90.
Findings: In this study, we identify tryptophan (TRP) as an agonist of PD-L1 induction through the AKT signaling pathway. TRP also synergistically enhanced PD-L1 expression on β cells exposed to interferon-γ. Conversely, interferon-γ-mediated induction of HLA-I and CXCL10 genes was down-regulated upon TRP treatment. Finally, TRP and its derivatives inhibited the activation of islet-reactive CD8+ T cells by β cells.
Interpretation: Collectively, our findings indicate that TRP could induce immune tolerance to β cells by promoting their immune evasion through HLA-I downregulation and PD-L1 upregulation.
Funding: Dutch Diabetes Research Foundation, DON Foundation, the Laboratoire d'Excellence consortium Revive (ANR-10-LABX-0073), Agence Nationale de la Recherche (ANR-19-CE15-0014-01), Fondation pour la Recherche Médicale (EQ U201903007793-EQU20193007831), Innovative Medicines InitiativeINNODIA and INNODIA HARVEST, Aides aux Jeunes Diabetiques (AJD) and Juvenile Diabetes Research Foundation Ltd (JDRF).
Keywords: CD274; CXCL10; HLA-I; Immune checkpoint inhibitor; Islet; PD-L1; Pancreatic beta cells; Tryptophan; Type 1 diabetes.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

