Cell-type specific and multiscale dynamics of human focal seizures in limbic structures
- PMID: 37536281
- PMCID: PMC10689922
- DOI: 10.1093/brain/awad262
Cell-type specific and multiscale dynamics of human focal seizures in limbic structures
Abstract
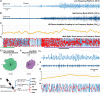
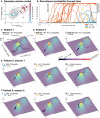
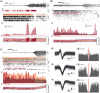
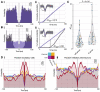
The relationship between clinically accessible epileptic biomarkers and neuronal activity underlying the transition to seizure is complex, potentially leading to imprecise delineation of epileptogenic brain areas. In particular, the pattern of interneuronal firing at seizure onset remains under debate, with some studies demonstrating increased firing and others suggesting reductions. Previous study of neocortical sites suggests that seizure recruitment occurs upon failure of inhibition, with intact feedforward inhibition in non-recruited territories. We investigated whether the same principle applies in limbic structures. We analysed simultaneous electrocorticography (ECoG) and neuronal recordings of 34 seizures in a cohort of 19 patients (10 male, 9 female) undergoing surgical evaluation for pharmacoresistant focal epilepsy. A clustering approach with five quantitative metrics computed from ECoG and multiunit data was used to distinguish three types of site-specific activity patterns during seizures, which at times co-existed within seizures. Overall, 156 single units were isolated, subclassified by cell-type and tracked through the seizure using our previously published methods to account for impacts of increased noise and single-unit waveshape changes caused by seizures. One cluster was closely associated with clinically defined seizure onset or spread. Entrainment of high-gamma activity to low-frequency ictal rhythms was the only metric that reliably identified this cluster at the level of individual seizures (P < 0.001). A second cluster demonstrated multi-unit characteristics resembling those in the first cluster, without concomitant high-gamma entrainment, suggesting feedforward effects from the seizure. The last cluster captured regions apparently unaffected by the ongoing seizure. Across all territories, the majority of both excitatory and inhibitory neurons reduced (69.2%) or ceased firing (21.8%). Transient increases in interneuronal firing rates were rare (13.5%) but showed evidence of intact feedforward inhibition, with maximal firing rate increases and waveshape deformations in territories not fully recruited but showing feedforward activity from the seizure, and a shift to burst-firing in seizure-recruited territories (P = 0.014). This study provides evidence for entrained high-gamma activity as an accurate biomarker of ictal recruitment in limbic structures. However, reduced neuronal firing suggested preserved inhibition in mesial temporal structures despite simultaneous indicators of seizure recruitment, in contrast to the inhibitory collapse scenario documented in neocortex. Further study is needed to determine if this activity is ubiquitous to hippocampal seizures or indicates a 'seizure-responsive' state in which the hippocampus is not the primary driver. If the latter, distinguishing such cases may help to refine the surgical treatment of mesial temporal lobe epilepsy.
Keywords: ictal recruitment; ictal territories; inhibition; interneurons; single unit.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
G.M.M. is an investigator and on the publication committee for the ‘Stereotactic Laser Ablation in Temporal Epilepsy’ (SLATE) trial funded by Medtronic, plc. None of the patients in this study were enrolled in the SLATE trial. The other authors report no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

