Inhibitory and excitatory synaptic neuroadaptations in the diazepam tolerant brain
- PMID: 37536384
- PMCID: PMC10578451
- DOI: 10.1016/j.nbd.2023.106248
Inhibitory and excitatory synaptic neuroadaptations in the diazepam tolerant brain
Abstract
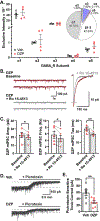
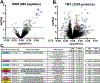
Benzodiazepine (BZ) drugs treat seizures, anxiety, insomnia, and alcohol withdrawal by potentiating γ2 subunit containing GABA type A receptors (GABAARs). BZ clinical use is hampered by tolerance and withdrawal symptoms including heightened seizure susceptibility, panic, and sleep disturbances. Here, we investigated inhibitory GABAergic and excitatory glutamatergic plasticity in mice tolerant to benzodiazepine sedation. Repeated diazepam (DZP) treatment diminished sedative effects and decreased DZP potentiation of GABAAR synaptic currents without impacting overall synaptic inhibition. While DZP did not alter γ2-GABAAR subunit composition, there was a redistribution of extrasynaptic GABAARs to synapses, resulting in higher levels of synaptic BZ-insensitive α4-containing GABAARs and a concomitant reduction in tonic inhibition. Conversely, excitatory glutamatergic synaptic transmission was increased, and NMDAR subunits were upregulated at synaptic and total protein levels. Quantitative proteomics further revealed cortex neuroadaptations of key pro-excitatory mediators and synaptic plasticity pathways highlighted by Ca2+/calmodulin-dependent protein kinase II (CAMKII), MAPK, and PKC signaling. Thus, reduced inhibitory GABAergic tone and elevated glutamatergic neurotransmission contribute to disrupted excitation/inhibition balance and reduced BZ therapeutic power with benzodiazepine tolerance.
Keywords: Benzodiazepine; GABA(A) receptor; NMDA receptor; Proteomics; Sedation; Tolerance.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no disclosures and declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

