Tumor Shape-Specific Brachytherapy Implants by 3D-Printing, Precision Radioactivity Painting, and Biomedical Imaging
- PMID: 37536742
- PMCID: PMC11468949
- DOI: 10.1002/adhm.202300528
Tumor Shape-Specific Brachytherapy Implants by 3D-Printing, Precision Radioactivity Painting, and Biomedical Imaging
Abstract
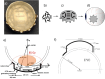
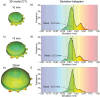
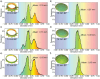
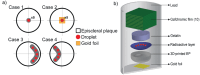
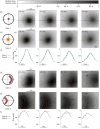
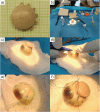
In brachytherapy (BT), or internal radiation therapy, cancer is treated by radioactive implants. For instance, episcleral plaques (EPs) for the treatment of uveal melanoma, are designed according to generic population approximations. However, more personalized implants can enhance treatment precision through better adjustment of dose profiles to the contours of cancerous tissues. An original approach integrating biomedical imaging, 3D printing, radioactivity painting, and biomedical imaging, is developed as a workflow for the development of tumor shape-specific BT implants. First, computer-aided design plans of EP are prepared according to guidelines prescribed by the Collaborative Ocular Melanoma Study protocol. Polyetheretherketone (PEEK), a high-performance polymer suitable for permanent implants, is used to 3D-print plaques and the geometrical accuracy of the printed design is evaluated by imaging. The possibility to modulate the dose distribution in a tridimensional manner is demonstrated by painting the inner surfaces of the EPs with radioactive 103Pd, followed by dose profile measurements. The possibility to modulate dose distributions generated by these 3D-printed plaques through radioactivity painting is therefore confirmed. Ex vivo surgical tests on human eyeballs are performed as an assessment of manipulation ease. Overall, this work provides a solution for the fabrication of tumor-specific radioactive implants requiring higher dose precision.
Keywords: 3D printing metrology; additive manufacturing of polymers; brachytherapy; brachytherapy dosimetry; episcleral plaques; polyetheretherketone (PEEK); uveal melanoma.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


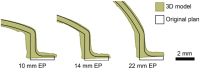

References
-
- a) Pei X., Zhang B., Fan Y., Zhu X., Sun Y., Wang Q., Zhang X., Zhou C., Mater. Lett. 2017, 208, 133;
- b) de Moraes P. H., Olate S., Cantín M., Assis A. F., Santos E., Silva F. d. O., Silva L. d. O., Int. J. Morphol. 2015, 33, 826;
- c) Murr L. E., Gaytan S. M., Medina F., Lopez H., Martinez E., Machado B. I., Hernandez D. H., Martinez L., Lopez M. I., Wicker R. B., Bracke J., Philos. Trans. R. Soc., A 2010, 368, 1999; - PubMed
- d) Ryan G., Pandit A., Apatsidis D. P., Biomaterials 2006, 27, 2651. - PubMed
-
- a) Bhargav A., Sanjairaj V., Rosa V., Feng L. W., Fuh Yh J., J. Biomed. Mater. Res., Part B 2018, 106, 2058; - PubMed
- b) Bibb R., Eggbeer D., Williams R., Rapid Prototyping J. 2006, 12, 95.
-
- a) Leong K. F., Cheah C. M., Chua C. K., Biomaterials 2003, 24, 2363; - PubMed
- b) Leong K. F., Chua C. K., Sudarmadji N., Yeong W. Y., J. Mech. Behav. Biomed. Mater. 2008, 1, 140; - PubMed
- c) Melchels F. P. W., Bertoldi K., Gabbrielli R., Velders A., Feijen J., Grijpma D. W., Biomaterials 2010, 31, 6909; - PubMed
- d) Zein I., Hutmacher D. W., Tan K. C., Teoh S. H., Biomaterials 2002, 23, 1169; - PubMed
- e) Shi J., Zhu L., Li L., Li Z., Yang J., Wang X., Sci. Rep. 2018, 8, 7395; - PMC - PubMed
- f) Tino R. B., Leary M., Yeo A. U., Kyriakou E., Kron T., Brandt M., Int. J. Extreme Manuf. 2020, 2, 012003;
- g) Poomathi N., Singh S., Prakash C., Subramanian A., Sahay R., Cinappan A., Ramakrishna S., Rapid Prototyping J. 2020, 26, 1313;
- h) Bose S., Ke D., Sahasrabudhe H., Bandyopadhyay A., Prog. Mater. Sci. 2018, 93, 45; - PMC - PubMed
- i) Li J. H., Wu C. T., Chu P. K., Gelinsky M., Mater. Sci. Eng., R 2020, 140, 100543;
- j) Vijayavenkataraman S., Yan W. C., Lu W. F., Wang C. H., Fuh J. Y. H., Adv. Drug Delivery Rev. 2018, 132, 296. - PubMed
-
- a) Song W. Y., Tanderup K., Pieters B., Emerging Technologies in Brachytherapy, CRC Press, Taylor & Francis Group, Boca Raton, FL: 2017;
- b) Tino R., Leary M., Yeo A., Kyriakou E., Kron T., Brandt M., Int. J. Extreme Manuf. 2020, 2, 012003.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

