Prevalence and clinical implications of germline pathogenic variants in cancer predisposing genes in young patients across sarcoma subtypes
- PMID: 37536918
- PMCID: PMC10803955
- DOI: 10.1136/jmg-2023-109269
Prevalence and clinical implications of germline pathogenic variants in cancer predisposing genes in young patients across sarcoma subtypes
Abstract
Background: Sarcomas are a rare and diverse group of cancers occurring mainly in young individuals for which an underlying germline genetic cause remains unclear in most cases.
Methods: Germline DNA from 177 children, adolescents and young adults with soft tissue or bone sarcomas was tested using multigene panels with 113 or 126 cancer predisposing genes (CPGs) to describe the prevalence of germline pathogenic/likely pathogenic variants (GPVs). Subsequent testing of a subset of tumours for loss of heterozygosity (LOH) evaluation was performed to investigate the clinical and molecular significance of these variants.
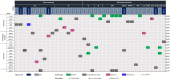
Results: GPVs were detected in 21.5% (38/177) of the patients (15.8% in children and 21.6% in adolescents and young adults), with dominant CPGs being altered in 15.2% overall. These variants were found in genes previously associated with the risk of developing sarcomas (TP53, RB1, NF1, EXT1/2) but also in genes where that risk is still emerging/limited (ERCC2, TSC2 and BRCA2) or unknown (PALB2, RAD50, FANCM and others). The detection rates of GPVs varied from 0% to 33% across sarcoma subtypes and GPV carriers were more likely to present more than one primary tumour than non-carriers (21.1%×6.5%; p=0.012). Loss of the wild-type allele was detected in 48% of tumours from GPV carriers, mostly in genes definitively associated with sarcoma risk.
Conclusion: Our findings reveal that a high proportion of young patients with sarcomas presented a GPV in a CPG, underscoring the urgency of establishing appropriate genetic screening strategies for these individuals and their families.
Keywords: genetic predisposition to disease; genetic variation; neoplasms.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
