Chronic immune-related adverse events in patients with cancer receiving immune checkpoint inhibitors: a systematic review
- PMID: 37536939
- PMCID: PMC10401216
- DOI: 10.1136/jitc-2022-006500
Chronic immune-related adverse events in patients with cancer receiving immune checkpoint inhibitors: a systematic review
Abstract
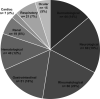
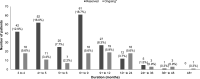
Immune-related adverse events (irAEs) are toxicities resulting from use of immune checkpoint inhibitors (ICIs). These side effects persist in some patients despite withholding therapy and using immunosuppressive and immune-modulating agents. Little is known about chronic irAEs and they are felt to be rare. We performed a systematic review to characterize non-endocrine chronic irAEs reported in the literature and describe their management. Ovid MEDLINE and Embase databases were searched for reports of adult patients with solid cancers treated with ICIs who experienced chronic (>12 weeks) non-endocrine irAEs. Patient, treatment and toxicity data were collected. Of 6843 articles identified, 229 studies including 323 patients met our inclusion criteria. The median age was 65 (IQR 56-72) and 58% were male. Most patients (75%) had metastatic disease and the primary cancer site was melanoma in 43% and non-small cell lung cancer in 31% of patients. The most common ICIs delivered were pembrolizumab (24%) and nivolumab (37%). The chronic irAEs experienced were rheumatological in 20% of patients, followed by neurological in 19%, gastrointestinal in 16% and dermatological in 14%. The irAE persisted for a median (range) of 180 (84-2370) days and 30% of patients had ongoing symptoms or treatment. More than half (52%) of patients had chronic irAEs that persisted for >6 months. The ICI was permanently discontinued in 60% of patients and 76% required oral and/or intravenous steroids. This is the first systematic review to assess and report on moderate/severe chronic non-endocrine irAEs after treatment with ICI in the literature. These toxicities persisted for months-years and the majority required discontinuation of therapy and initiation of immunosuppression. Further research is needed to better understand chronic irAEs, which hold potential substantial clinical significance considering the expanded use of ICIs and their integration into the (neo)adjuvant settings.
Keywords: Autoimmunity; Immune Checkpoint Inhibitors; Immunotherapy; Review.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: EM has received honoraria from Merck and Bristol Myers Squibb.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
