A porcine model of postoperative hemi-diaphragmatic paresis to evaluate a unilateral diaphragmatic pacemaker
- PMID: 37537216
- PMCID: PMC10400610
- DOI: 10.1038/s41598-023-39468-w
A porcine model of postoperative hemi-diaphragmatic paresis to evaluate a unilateral diaphragmatic pacemaker
Abstract
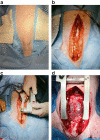
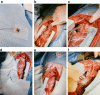
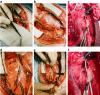
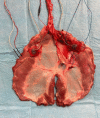
Unilateral phrenic nerve damage is a dreaded complication in congenital heart surgery. It has deleterious effects in neonates and children with uni-ventricular circulation. Diaphragmatic palsy, caused by phrenic nerve damage, impairs respiratory function, especially in new-borns, because their respiration depends on diaphragmatic contractions. Furthermore, Fontan patients with passive pulmonary perfusion are seriously affected by phrenic nerve injury, because diaphragmatic contraction augments pulmonary blood flow. Diaphragmatic plication is currently employed to ameliorate the negative effects of diaphragmatic palsy on pulmonary perfusion and respiratory mechanics. This procedure attenuates pulmonary compression by the abdominal contents. However, there is no contraction of the plicated diaphragm and consequently no contribution to the pulmonary blood flow. Hence, we developed a porcine model of unilateral diaphragmatic palsy in order to evaluate a diaphragmatic pacemaker. Our illustrated step-by-step description of the model generation enables others to replicate and use our model for future studies. Thereby, it might contribute to investigation and advancement of potential improvements for these patients.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

