Unique functional responses differentially map onto genetic subtypes of dopamine neurons
- PMID: 37537242
- PMCID: PMC10545540
- DOI: 10.1038/s41593-023-01401-9
Unique functional responses differentially map onto genetic subtypes of dopamine neurons
Abstract
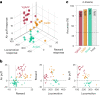
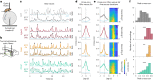
Dopamine neurons are characterized by their response to unexpected rewards, but they also fire during movement and aversive stimuli. Dopamine neuron diversity has been observed based on molecular expression profiles; however, whether different functions map onto such genetic subtypes remains unclear. In this study, we established that three genetic dopamine neuron subtypes within the substantia nigra pars compacta, characterized by the expression of Slc17a6 (Vglut2), Calb1 and Anxa1, each have a unique set of responses to rewards, aversive stimuli and accelerations and decelerations, and these signaling patterns are highly correlated between somas and axons within subtypes. Remarkably, reward responses were almost entirely absent in the Anxa1+ subtype, which instead displayed acceleration-correlated signaling. Our findings establish a connection between functional and genetic dopamine neuron subtypes and demonstrate that molecular expression patterns can serve as a common framework to dissect dopaminergic functions.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures















References
-
- Schultz W. The reward signal of midbrain dopamine neurons. N. Physiol. Sci. 1999;14:249–254. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

