Methionine restriction-induced sulfur deficiency impairs antitumour immunity partially through gut microbiota
- PMID: 37537369
- PMCID: PMC10513933
- DOI: 10.1038/s42255-023-00854-3
Methionine restriction-induced sulfur deficiency impairs antitumour immunity partially through gut microbiota
Abstract
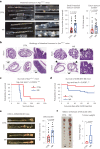
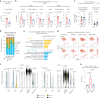
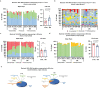
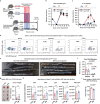
Restriction of methionine (MR), a sulfur-containing essential amino acid, has been reported to repress cancer growth and improve therapeutic responses in several preclinical settings. However, how MR impacts cancer progression in the context of the intact immune system is unknown. Here we report that while inhibiting cancer growth in immunocompromised mice, MR reduces T cell abundance, exacerbates tumour growth and impairs tumour response to immunotherapy in immunocompetent male and female mice. Mechanistically, MR reduces microbial production of hydrogen sulfide, which is critical for immune cell survival/activation. Dietary supplementation of a hydrogen sulfide donor or a precursor, or methionine, stimulates antitumour immunity and suppresses tumour progression. Our findings reveal an unexpected negative interaction between MR, sulfur deficiency and antitumour immunity and further uncover a vital role of gut microbiota in mediating this interaction. Our study suggests that any possible anticancer benefits of MR require careful consideration of both the microbiota and the immune system.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures










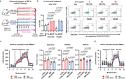
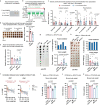

References
-
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J. Nutr. 1993;123:269–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

