Comparing the Safety Action Feedback and Engagement (SAFE) Loop with an established incident reporting system: Study protocol for a pragmatic cluster randomized controlled trial
- PMID: 37538195
- PMCID: PMC10393596
- DOI: 10.1016/j.conctc.2023.101192
Comparing the Safety Action Feedback and Engagement (SAFE) Loop with an established incident reporting system: Study protocol for a pragmatic cluster randomized controlled trial
Abstract
Background: Incident reporting is widely used in hospitals to improve patient safety, but current reporting systems do not function optimally. The utility of incident reports is limited because hospital staff may not know what to report, may fear retaliation, and may doubt whether administrators will review reports and respond effectively.
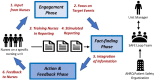
Methods: This is a clustered randomized controlled trial of the Safety Action Feedback and Engagement (SAFE) Loop, an intervention designed to transform hospital incident reporting systems into effective tools for improving patient safety. The SAFE Loop has six key attributes: obtaining nurses' input about which safety problems to prioritize on their unit; focusing on learning about selected high-priority events; training nurses to write more informative event reports; prompting nurses to report high-priority events; integrating information about events from multiple sources; and providing feedback to nurses on findings and mitigation plans. The study will focus on medication errors and randomize 20 nursing units at a large academic/community hospital in Los Angeles. Outcomes include: (1) incident reporting practices (rates of high-priority reports, contributing factors described in reports), (2) nurses' attitudes toward incident reporting, and (3) rates of high-priority events. Quantitative analyses will compare changes in outcomes pre- and post-implementation between the intervention and control nursing units, and qualitative analyses will explore nurses' experiences with implementation.
Conclusion: If effective, SAFE Loop will have several benefits: increasing nurses' engagement with reporting, producing more informative reports, enabling safety leaders to understand problems, designing system-based solutions more effectively, and lowering rates of high-priority patient safety events.
Keywords: Hospital incident reporting; Human factors engineering; Medical errors; Nursing care; Patient safety; Randomized controlled trial.
© 2023 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Agency for Healthcare Research and Quality . Agency for Healthcare Research and Quality; 2019. Medication Errors and Adverse Drug Events.https://psnet.ahrq.gov/primers/primer/23/Medication-Errors-and-Adverse-D... Patient Safety Network Web site. . Published 2019. Updated 1.
-
- Woods D. Enhancing Patient Safety and Reducing Errors in Health Care; Chicago: 1999. Improving the Reporting and Analysis of Incidents.
-
- Nuckols T.K., Bell D.S., Paddock S.M., Hilborne L.H. Contributing factors identified by hospital incident report narratives. Qual. Saf. Health Care. 2008;17(5):368–372. - PubMed
-
- Nuckols T. vol. 2019. Agency for Healthcare Research and Quality; 2011. Incident reporting: more attention to the safety action feedback Loop, please. (Perspectives on Safety).