Dose- and application route-dependent effects of betahistine on behavioral recovery and neuroplasticity after acute unilateral labyrinthectomy in rats
- PMID: 37538257
- PMCID: PMC10395078
- DOI: 10.3389/fneur.2023.1175481
Dose- and application route-dependent effects of betahistine on behavioral recovery and neuroplasticity after acute unilateral labyrinthectomy in rats
Abstract
Introduction: Betahistine is widely used for the treatment of various vestibular disorders. However, the approved oral administration route and maximum daily dose are evidently not effective in clinical trials, possibly due to a major first-pass metabolism by monoamine oxidases (MAOs). The current study aimed to test different application routes (i.v./s.c./p.o.), doses, and concurrent medication (with the MAO-B inhibitor selegiline) for their effects on behavioral recovery and cerebral target engagement following unilateral labyrinthectomy (UL) in rats.
Methods: Sixty rats were subjected to UL by transtympanic injection of bupivacaine/arsanilic acid and assigned to five treatment groups: i.v. low-dose betahistine (1 mg/kg bid), i.v. high-dose betahistine (10 mg/kg bid), p.o. betahistine (1 mg/kg bid)/selegiline (1 mg/kg once daily), s.c. betahistine (continuous release of 4.8 mg/day), and i.v. normal saline bid (sham treatment; days 1-3 post-UL), respectively. Behavioral testing of postural asymmetry, nystagmus, and mobility in an open field was performed seven times until day 30 post-UL and paralleled by sequential cerebral [18F]-FDG-μPET measurements.
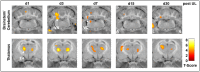
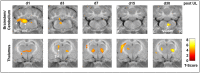
Results: The therapeutic effects of betahistine after UL differed in extent and time course and were dependent on the dose, application route, and selegiline co-medication: Postural asymmetry was significantly reduced on 2-3 days post-UL by i.v. high-dose and s.c. betahistine only. No changes were observed in the intensity of nystagmus across groups. When compared to sham treatment, movement distance in the open field increased up to 5-fold from 2 to 30 days post-UL in the s.c., i.v. high-dose, and p.o. betahistine/selegiline groups. [18F]-FDG-μPET showed a dose-dependent rCGM increase in the ipsilesional vestibular nucleus until day 3 post-UL for i.v. high- vs. low-dose betahistine and sham treatment, as well as for p.o. betahistine/selegiline and s.c. betahistine vs. sham treatment. From 1 to 30 days post-UL, rCGM increased in the thalamus bilaterally for i.v. high-dose betahistine, s.c. betahistine, and p.o. betahistine/selegiline vs. saline treatment.
Discussion: Betahistine has the potential to augment the recovery of dynamic deficits after UL if the administration protocol is optimized toward higher effective plasma levels. This may be achieved by higher doses, inhibition of MAO-based metabolism, or a parenteral route. In vivo imaging suggests a drug-target engagement in central vestibular networks.
Keywords: Menière's disease; acute unilateral vestibulopathy; animal models; betahistine; neuroimaging; vestibular disorders.
Copyright © 2023 Antons, Lindner, Eilles, Günther, Delker, Branner, Krämer, Beck, Oos, Wuehr, Ziegler, Strupp and Zwergal.
Conflict of interest statement
MS is a Joint Chief Editor of the Journal of Neurology, Editor in Chief of Frontiers of Neuro-otology, and Section Editor of F1000. He has received speaker's honoraria from Abbott, Auris Medical, Biogen, Eisai, Grünenthal, GSK, Henning Pharma, Interacoustics, J&J, MSD, NeuroUpdate, Otometrics, Pierre-Fabre, TEVA, UCB, and Viatris. He receives support for clinical studies from Decibel, USA, Cure within Reach, USA, and Heel, Germany. He distributes M-glasses and Positional vertigo App. He acts as a consultant for Abbott, AurisMedical, Bulbitec, Heel, IntraBio, Sensorion, and Vertify. He is an investor and shareholder of IntraBio. AZ is an Associate Editor of Frontiers in Neuro-otology and Frontiers in Translational Rehabilitation Sciences, as well as Guest Editor of the Journal of Neurology. He has received speaker's honoraria and research support from Dr. Willmar Schwabe GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Ginkgo biloba Extract EGb 761 Improves Vestibular Compensation and Modulates Cerebral Vestibular Networks in the Rat.Front Neurol. 2019 Feb 25;10:147. doi: 10.3389/fneur.2019.00147. eCollection 2019. Front Neurol. 2019. PMID: 30858822 Free PMC article.
-
N-acetyl-L-leucine accelerates vestibular compensation after unilateral labyrinthectomy by action in the cerebellum and thalamus.PLoS One. 2015 Mar 24;10(3):e0120891. doi: 10.1371/journal.pone.0120891. eCollection 2015. PLoS One. 2015. PMID: 25803613 Free PMC article.
-
In Vivo Imaging of Glial Activation after Unilateral Labyrinthectomy in the Rat: A [18F]GE180-PET Study.Front Neurol. 2017 Dec 11;8:665. doi: 10.3389/fneur.2017.00665. eCollection 2017. Front Neurol. 2017. PMID: 29312111 Free PMC article.
-
Vestibular compensation: Neural mechanisms and clinical implications for the treatment of vertigo.Auris Nasus Larynx. 2024 Apr;51(2):328-336. doi: 10.1016/j.anl.2023.11.009. Epub 2023 Dec 19. Auris Nasus Larynx. 2024. PMID: 38114342 Review.
-
In vivo neuroplasticity in vestibular animal models.Mol Cell Neurosci. 2022 May;120:103721. doi: 10.1016/j.mcn.2022.103721. Epub 2022 Mar 22. Mol Cell Neurosci. 2022. PMID: 35338004 Review.
Cited by
-
Examination of betahistine bioavailability in combination with the monoamine oxidase B inhibitor, selegiline, in humans-a non-randomized, single-sequence, two-period titration, open label single-center phase 1 study (PK-BeST).Front Neurol. 2023 Oct 18;14:1271640. doi: 10.3389/fneur.2023.1271640. eCollection 2023. Front Neurol. 2023. PMID: 37920833 Free PMC article.
-
Histaminergic System and Vestibular Function in Normal and Pathological Conditions.Curr Neuropharmacol. 2024;22(11):1826-1845. doi: 10.2174/1570159X22666240319123151. Curr Neuropharmacol. 2024. PMID: 38504566 Free PMC article. Review.
-
Prevalence of vestibular disease in France: analysis of prescription data from a national health insurance database.J Neurol. 2024 Aug;271(8):4865-4870. doi: 10.1007/s00415-024-12423-z. Epub 2024 May 10. J Neurol. 2024. PMID: 38727733 Free PMC article.
References
LinkOut - more resources
Full Text Sources

