Supramolecular assemblies in the molecular complexes of 4-cyanophenylboronic acid with different N-donor ligands
- PMID: 37538513
- PMCID: PMC10394587
- DOI: 10.1039/d3ra03936f
Supramolecular assemblies in the molecular complexes of 4-cyanophenylboronic acid with different N-donor ligands
Abstract
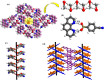
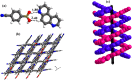
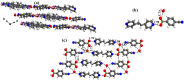
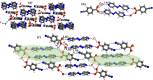
Molecular complexes of 4-cyanophenylboronic acid (CB) with various N-donor compounds having different conformational features, for example, rigid (1,10-phenanthroline (110phen), 4,7-phenanthroline (47phen), 1,7-phenanthroline (17phen) and acridine (acr)) and linear (1,2-bis(4-pyridyl)ethane (bpyea), 1,2-bis(4-pyridyl)ethene (bpyee) and 4,4'-azopyridine (azopy)), have been reported. In all complexes, the -B(OH)2 moiety is found to be in a syn-anti confirmation, with the exception of structures containing 110phen, bpyee, and azopy, wherein, syn-syn conformation is observed. Further, CB molecules remain intact in all structures except in the complexes with some linear N-donor ligands, wherein -B(OH)2 transforms to monoester (-B(OH)(OCH3)) prior to the formation of corresponding molecular complexes. In such boronic monoester complexes, the conformation of -B(OH)(OCH3) is syn-anti with respect to the -OH and -OCH3 groups. Also, complexes mediated by azopy and bpyee exist in both hydrated and anhydrous forms. In these anhydrous structures, the recognition pattern is through homomeric (juxtaposed -CN and -B(OH)2) as well as heteromeric (between hetero N-atom and -B(OH)2) O-H⋯N hydrogen bonds, while only heteromeric O-H⋯N hydrogen bonds hold co-formers in all other structures. Depending upon the conformational features of both co-formers, molecules are packed in crystal lattices in the form of stacked layers, helical chains, and crossed ribbons. All structures are fully characterized by single-crystal X-ray diffraction and phase purity is established by powder X-ray diffraction. Additionally, correlation among structures is explained by calculating a similarity index and performing a Hirshfeld surface analysis to quantify the strength and effectiveness of different types of intermolecular bonds that stabilize these structures along with the presentation of energy frameworks, representing the strength of the interactions in the form gradient cylinders. Also, the morphology of each complex was computed by BFDH methodology to correlate with the actual crystal morphology and packing arrangement.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Systematic Exploration of Structural Topologies in Hydrogen-Bonded Supramolecular Assemblies of Citric Acid with Different Heterocyclic Compounds.ACS Omega. 2023 Jun 15;8(25):23202-23217. doi: 10.1021/acsomega.3c03446. eCollection 2023 Jun 27. ACS Omega. 2023. PMID: 37396223 Free PMC article.
-
A competitive molecular recognition study: syntheses and analysis of supramolecular assemblies of 3,5-dihydroxybenzoic acid and its bromo derivative with some N-donor compounds.Chemistry. 2006 Feb 8;12(6):1597-609. doi: 10.1002/chem.200500570. Chemistry. 2006. PMID: 16250059
-
Tetrel Bonding and Other Non-Covalent Interactions Assisted Supramolecular Aggregation in a New Pb(II) Complex of an Isonicotinohydrazide.Molecules. 2020 Sep 4;25(18):4056. doi: 10.3390/molecules25184056. Molecules. 2020. PMID: 32899863 Free PMC article.
-
A rational study of crystal engineering of supramolecular assemblies of 1,2,4,5-benzenetetracarboxylic acid.J Org Chem. 2003 Nov 28;68(24):9177-85. doi: 10.1021/jo034434z. J Org Chem. 2003. PMID: 14629133
-
Syntheses and crystal structures of a series of coordination polymers constructed from C2-symmetric ligand 1,3-adamantanedicarboxylic acid.Chem Asian J. 2010 Jul 5;5(7):1611-9. doi: 10.1002/asia.200900751. Chem Asian J. 2010. PMID: 20533433
References
-
- MacDonald J. C. Whitesides G. M. Chem. Rev. 1994;94:2383–2420.
- Braga D. and Grepioni F., Crystal Engineering: from Molecules and Crystals to Materials, Springer, 1999
- Alivisatos P. Barbara P. F. Castleman A. W. Chang J. Dixon D. A. Klein M. L. McLendon G. L. Miller J. S. Ratner M. A. Rossky P. J. Adv. Mater. 1998;10:1297–1336.
- Atwood J. L., Comprehensive Supramolecular Chemistry II, Elsevier, 2017
-
- Desiraju G. R. Nature. 2001;412:397–400. - PubMed
- Sun L. Zhu W. Zhang X. Li L. Dong H. Hu W. J. Am. Chem. Soc. 2021;143:19243–19256. - PubMed
- Braga D. Grepioni F. Acc. Chem. Res. 2000;33:601–608. - PubMed
- Bernstein J. Novoa J. J. Boese R. Cirkel S. A. Chem.–Eur. J. 2010;16:9047–9055. - PubMed
- Ahn S. PrakashaReddy J. Kariuki B. M. Chatterjee S. Ranganathan A. Pedireddi V. R. Rao C. N. R. Harris K. D. M. Chem.–Eur. J. 2005;11:2433–2439. - PubMed
-
- Li P. Ryder M. R. Stoddart J. F. Acc. Mater. Res. 2020;1:77–87.
- Eccles K. S. Morrison R. E. Maguire A. R. Lawrence S. E. Cryst. Growth Des. 2014;14:2753–2762.
- Easmin S. Pedireddi V. R. Cryst. Growth Des. 2023;23:2802–2811.
-
- Pereira Silva P. S. Castro R. A. E. Melro E. Silva M. R. Maria T. M. R. Canotilho J. Eusébio M. E. S. J. Therm. Anal. Calorim. 2015;120:667–677.
- Nowak M. Dyba A. J. Janczak J. Morritt A. Fábián L. Karolewicz B. Khimyak Y. Z. Braun D. E. Nartowski K. P. Mol. Pharm. 2022;19:456–471. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

