EGFR Suppression Inhibits the Sphere Formation of MCF7 Cells Overexpressing EGFR
- PMID: 37538799
- PMCID: PMC10395776
- DOI: 10.32607/actanaturae.17857
EGFR Suppression Inhibits the Sphere Formation of MCF7 Cells Overexpressing EGFR
Abstract
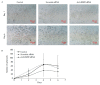
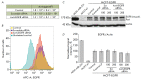
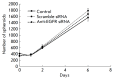
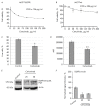
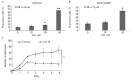
The epidermal growth factor receptor (EGFR) is an oncogenic tyrosine kinase that is involved in tumor initiation and progression, making EGFR inhibitors and monoclonal antibodies to this receptor essential for anti-tumor therapy. We have previously shown that EGFR transgene expression in the human breast adenocarcinoma cell line MCF7 (MCF7-EGFR) stimulates the 3D spheroid-like growth. The primary focus of our present work was to investigate whether EGFR inhibition could affect the assembly of spheroids or lead to the destruction of pre-existing spheroids. We compared the effects of anti-EGFR siRNA, the anti-EGFR monoclonal antibody cetuximab, and the tyrosine kinase inhibitor AG1478 on dissociated and spheroid MCF7-EGFR cells. MCF7-EGFR cells were found to have a 2.5-fold higher sensitivity towards the cytotoxic effects of cetuximab and AG1478 compared with the parental MCF7 cell line. The suppression of EGFR mRNA with siRNA was found to reduce the sphere formation, whereas treating the pre-existing spheroids had no such effect. Treatment of dissociated spheroids with cetuximab and AG1478 was also found to inhibit the MCF7-EGFR sphere formation. We suggest that EGFR expression is important, at least, during the spheroid formation stage. The transition of a MCF7wt adherent cell culture to MCF7-EGFR spheroids was accompanied by a considerable increase in N-cadherin adhesion proteins. The level of N-cadherin decreased when MCF7-EGFR cells were treated with siRNA and cetuximab. Thus, we have demonstrated that N-cadherin is involved in the EGFR-dependent formation of MCF7-EGFR spheroids. Accordingly, MCF7-EGFR spheroids can be considered a suitable model for studying aggressive hormone-positive breast tumors.
Keywords: 3D cell culture; AG1478; EGFR; MCF7; cetuximab; siRNA; spheroids.
Copyright ® 2023 National Research University Higher School of Economics.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
