Establishing a single-sex controlled human Schistosoma mansoni infection model for Uganda: protocol for safety and dose-finding trial
- PMID: 37538934
- PMCID: PMC10396375
- DOI: 10.1093/immadv/ltad010
Establishing a single-sex controlled human Schistosoma mansoni infection model for Uganda: protocol for safety and dose-finding trial
Abstract
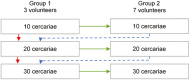
Control of schistosomiasis depends on a single drug, praziquantel, with variable cure rates, high reinfection rates, and risk of drug resistance. A vaccine could transform schistosomiasis control. Preclinical data show that vaccine development is possible, but conventional vaccine efficacy trials require high incidence, long-term follow-up, and large sample size. Controlled human infection studies (CHI) can provide early efficacy data, allowing the selection of optimal candidates for further trials. A Schistosoma CHI has been established in the Netherlands but responses to infection and vaccines differ in target populations in endemic countries. We aim to develop a CHI for Schistosoma mansoni in Uganda to test candidate vaccines in an endemic setting. This is an open-label, dose-escalation trial in two populations: minimal, or intense, prior Schistosoma exposure. In each population, participants will be enrolled in sequential dose-escalating groups. Initially, three volunteers will be exposed to 10 cercariae. If all show infection, seven more will be exposed to the same dose. If not, three volunteers in subsequent groups will be exposed to higher doses (20 or 30 cercariae) following the same algorithm, until all 10 volunteers receiving a particular dose become infected, at which point the study will be stopped for that population. Volunteers will be followed weekly after infection until CAA positivity or to 12 weeks. Once positive, they will be treated with praziquantel and followed for one year. The trial registry number is ISRCTN14033813 and all approvals have been obtained. The trial will be subjected to monitoring, inspection, and/or audits.
Keywords: human-controlled Schistosoma masoni.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
The authors declare that the research was conceptualized and protocol written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
