Colistin Monotherapy versus Combination Therapy for Carbapenem-Resistant Organisms
- PMID: 37538951
- PMCID: PMC10398788
- DOI: 10.1056/evidoa2200131
Colistin Monotherapy versus Combination Therapy for Carbapenem-Resistant Organisms
Abstract
Background: Pneumonia and bloodstream infections (BSI) due to extensively drug-resistant (XDR) Acinetobacter baumannii, XDR Pseudomonas aeruginosa, and carbapenem-resistant Enterobacterales (CRE) are associated with high mortality rates, and therapeutic options remain limited. This trial assessed whether combination therapy with colistin and meropenem was superior to colistin monotherapy for the treatment of these infections.
Methods: The OVERCOME (Colistin Monotherapy versus Combination Therapy) trial was an international, randomized, double-blind, placebo-controlled trial. We randomly assigned participants to receive colistin (5 mg/kg once followed by 1.67 mg/kg every 8 hours) in combination with either meropenem (1000 mg every 8 hours) or matching placebo for the treatment of pneumonia and/or BSI caused by XDR A. baumannii, XDR P. aeruginosa, or CRE. The primary outcome was 28-day mortality, and secondary outcomes included clinical failure and microbiologic cure.
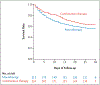
Results: Between 2012 and 2020, a total of 464 participants were randomly assigned to treatment, and 423 eligible patients comprised the modified intention-to-treat population. A. baumannii was the predominant trial pathogen (78%) and pneumonia the most common index infection (70%). Most patients were in the intensive care unit at the time of enrollment (69%). There was no difference in mortality (43 vs. 37%; P=0.17), clinical failure (65 vs. 58%; difference, 6.8 percentage points; 95% confidence interval [CI], -3.1 to 16.6), microbiologic cure (65 vs. 60%; difference, 4.8 percentage points; 95% CI, -5.6 to 15.2), or adverse events (acute kidney injury, 52 vs. 49% [P=0.55]; hypersensitivity reaction, 1 vs. 3% [P=0.22]; and neurotoxicity, 5 vs. 2% [P=0.29]) between patients receiving monotherapy and combination therapy, respectively.
Conclusions: Combination therapy with colistin and meropenem was not superior to colistin monotherapy for the treatment of pneumonia or BSI caused by these pathogens. (Funded by the National Institute of Allergy and Infectious Diseases, Division of Microbiology and Infectious Diseases protocol 10-0065; ClinicalTrials.gov number, NCT01597973.).
Figures

Comment in
-
Colistin - That Was Fun, But Now We're Done.NEJM Evid. 2023 Jan;2(1):EVIDe2200298. doi: 10.1056/EVIDe2200298. Epub 2022 Dec 27. NEJM Evid. 2023. PMID: 38320089
References
-
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. December 2019. U.S. Department of Health and Human Services; (www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508...).
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
