Understanding NK cell biology for harnessing NK cell therapies: targeting cancer and beyond
- PMID: 37539051
- PMCID: PMC10395517
- DOI: 10.3389/fimmu.2023.1192907
Understanding NK cell biology for harnessing NK cell therapies: targeting cancer and beyond
Abstract
Gene-engineered immune cell therapies have partially transformed cancer treatment, as exemplified by the use of chimeric antigen receptor (CAR)-T cells in certain hematologic malignancies. However, there are several limitations that need to be addressed to target more cancer types. Natural killer (NK) cells are a type of innate immune cells that represent a unique biology in cancer immune surveillance. In particular, NK cells obtained from heathy donors can serve as a source for genetically engineered immune cell therapies. Therefore, NK-based therapies, including NK cells, CAR-NK cells, and antibodies that induce antibody-dependent cellular cytotoxicity of NK cells, have emerged. With recent advances in genetic engineering and cell biology techniques, NK cell-based therapies have become promising approaches for a wide range of cancers, viral infections, and senescence. This review provides a brief overview of NK cell characteristics and summarizes diseases that could benefit from NK-based therapies. In addition, we discuss recent preclinical and clinical investigations on the use of adoptive NK cell transfer and agents that can modulate NK cell activity.
Keywords: aging; cancer; chimeric antigen receptor; immune surveillance; immunotherapy; natural killer cell.
Copyright © 2023 Shin, Bak, Park, Kim, Yoon, Jung and Noh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
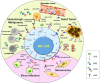
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

