ChemR23 activation reprograms macrophages toward a less inflammatory phenotype and dampens carcinoma progression
- PMID: 37539056
- PMCID: PMC10396772
- DOI: 10.3389/fimmu.2023.1196731
ChemR23 activation reprograms macrophages toward a less inflammatory phenotype and dampens carcinoma progression
Abstract
Introduction: Tumor Associated Macrophages (TAM) are a major component of the tumor environment and their accumulation often correlates with poor prognosis by contributing to local inflammation, inhibition of anti-tumor immune response and resistance to anticancer treatments. In this study, we thus investigated the anti-cancer therapeutic interest to target ChemR23, a receptor of the resolution of inflammation expressed by macrophages, using an agonist monoclonal antibody, αChemR23.
Methods: Human GM-CSF, M-CSF and Tumor Associated Macrophage (TAM)-like macrophages were obtained by incubation of monocytes from healthy donors with GM-CSF, M-CSF or tumor cell supernatants (Breast cancer (BC) or malignant pleural mesothelioma (MPM) cells). The effects of αChemR23 on macrophages were studied at the transcriptomic, protein and functional level. Datasets from The Cancer Genome Atlas (TCGA) were used to study CMKLR1 expression, coding for ChemR23, in BC and MPM tumors. In vivo, αChemR23 was evaluated on overall survival, metastasis development and transcriptomic modification of the metastatic niche using a model of resected triple negative breast cancer.
Results: We show that ChemR23 is expressed at higher levels in M-CSF and tumor cell supernatant differentiated macrophages (TAM-like) than in GM-CSF-differentiated macrophages. ChemR23 activation triggered by αChemR23 deeply modulates M-CSF and TAM-like macrophages including profile of cell surface markers, cytokine secretion, gene mRNA expression and immune functions. The expression of ChemR23 coding gene (CMKLR1) strongly correlates to TAM markers in human BC tumors and MPM and its histological detection in these tumors mainly corresponds to TAM expression. In vivo, treatment with αChemR23 agonist increased mouse survival and decreased metastasis occurrence in a model of triple-negative BC in correlation with modulation of TAM phenotype in the metastatic niche.
Conclusion: These results open an attractive opportunity to target TAM and the resolution of inflammation pathways through ChemR23 to circumvent TAM pro-tumoral effects.
Keywords: ChemR23 receptor; agonist; antibody; cancer; macrophage.
Copyright © 2023 Lavy, Gauttier, Dumont, Chocteau, Deshayes, Fresquet, Dehame, Girault, Trilleaud, Neyton, Mary, Juin, Poirier, Barillé-Nion and Blanquart.
Conflict of interest statement
CT, VG, CM, and NP are inventors on a patent application (no. WO 2019/193029, filed 3 April 2019, published 8 October 2019) and product (WO2021/069709, filed 09 october 2019, published 15 April 2021) related to this work filed by OSE Immunotherapeutics and employees of OSE Immunotherapeutics with IG and SN. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This study was partially supported by OSE Immunotherapeutics funding.
Figures

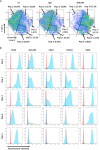

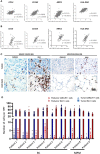
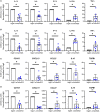


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

