Potential drug-drug interactions among U.S. adults treated with nirmatrelvir/ritonavir: A cross-sectional study of the National Covid Cohort Collaborative (N3C)
- PMID: 37539477
- PMCID: PMC10838345
- DOI: 10.1002/phar.2860
Potential drug-drug interactions among U.S. adults treated with nirmatrelvir/ritonavir: A cross-sectional study of the National Covid Cohort Collaborative (N3C)
Abstract
Study objective: To estimate the prevalence of potential moderate to severe drug-drug interactions (DDIs) involving nirmatrelvir/ritonavir, identify interacting medications, and evaluate risk factors associated with potential DDIs.
Design: Cross-sectional study.
Data source: Electronic health records from the National COVID Cohort Collaborative Enclave, one of the largest COVID-19 data resources in the United States.
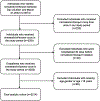
Patients: Outpatients aged ≥18 years and started nirmatrelvir/ritonavir between December 23, 2021 and March 31, 2022.
Intervention: Nirmatrelvir/ritonavir.
Measurements: The outcome is potential moderate to severe DDIs, defined as starting interacting medications reported by National Institutes of Health 30 days before or 10 days after starting nirmatrelvir/ritonavir.
Main results: Of 3214 outpatients who started nirmatrelvir/ritonavir, the mean age was 56.8 ± 17.1 years, 39.5% were male, and 65.8% were non-Hispanic white. Overall, 521 (16.2%) were potentially exposed to at least one moderate to severe DDI, most commonly to atorvastatin (19.2% of all DDIs), hydrocodone (14.0%), or oxycodone (14.0%). After adjustment for covariates, potential DDIs were more likely among individuals who were older (odds ratio [OR] 1.16 per 10-year increase, 95% confidence interval [CI] 1.08-1.25), male (OR 1.36, CI 1.09-1.71), smokers (OR 1.38, CI 1.10-1.73), on more co-medications (OR 1.35, CI 1.31-1.39), and with a history of solid organ transplant (OR 3.63, CI 2.05-6.45).
Conclusions: One in six of individuals receiving nirmatrelvir/ritonavir were at risk of a potential moderate or severe DDI, underscoring the importance of clinical and pharmacy systems to mitigate such risks.
Keywords: SARS-CoV-2; antivirals; drug interactions; drug safety; nirmatrelvir/ritonavir; patient safety.
© 2023 Pharmacotherapy Publications, Inc.
Conflict of interest statement
Figures

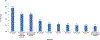

References
-
- Centers for Disease Control and Prevention. COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2023, April 03. https://covid.cdc.gov/covid-data-tracker
-
- Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19 [Internet]. U.S.Food & Drug Administration; c2021[cited 2023 Mar 22]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...
-
- Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study [Internet]. Pfizer; c2021. [cited 2022 July 23]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-n....
-
- COVID-19 Treatment Guidelines [Internet]. National Institutes of Health; c2022. [cited 2022 May 29]. Available from: https://www.covid19treatmentguidelines.nih.gov/.
-
- COVID-19 Drug Interactions [Internet]. University of Liverpool; c2022. [cited 2022 May 10]. Available from: https://www.covid19-druginteractions.org/checker.
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR002649/TR/NCATS NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- UL1 TR001860/TR/NCATS NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- U54 GM104942/GM/NIGMS NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- UL1 TR001439/TR/NCATS NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- UL1 TR001445/TR/NCATS NIH HHS/United States
- UL1 TR003096/TR/NCATS NIH HHS/United States
- UM1 TR004556/TR/NCATS NIH HHS/United States
- U54 GM104938/GM/NIGMS NIH HHS/United States
- UL1 TR002537/TR/NCATS NIH HHS/United States
- UL1 TR001412/TR/NCATS NIH HHS/United States
- UL1 TR001872/TR/NCATS NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- UL1 TR002529/TR/NCATS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- UL1 TR002494/TR/NCATS NIH HHS/United States
- UL1 TR002736/TR/NCATS NIH HHS/United States
- U54 GM115516/GM/NIGMS NIH HHS/United States
- UL1 TR002369/TR/NCATS NIH HHS/United States
- UL1 TR002541/TR/NCATS NIH HHS/United States
- U54 GM115371/GM/NIGMS NIH HHS/United States
- UL1 TR002001/TR/NCATS NIH HHS/United States
- UL1 TR002538/TR/NCATS NIH HHS/United States
- U54 GM115458/GM/NIGMS NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- UL1 TR002535/TR/NCATS NIH HHS/United States
- UL1 TR001866/TR/NCATS NIH HHS/United States
- UL1 TR003167/TR/NCATS NIH HHS/United States
- UL1 TR001409/TR/NCATS NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- UL1 TR001453/TR/NCATS NIH HHS/United States
- UL1 TR002489/TR/NCATS NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- UL1 TR003107/TR/NCATS NIH HHS/United States
- UL1 TR003015/TR/NCATS NIH HHS/United States
- UL1 TR002733/TR/NCATS NIH HHS/United States
- UL1 TR001433/TR/NCATS NIH HHS/United States
- K01 AG070329/AG/NIA NIH HHS/United States
- U24 TR002306/TR/NCATS NIH HHS/United States
- UL1 TR002003/TR/NCATS NIH HHS/United States
- UL1 TR001876/TR/NCATS NIH HHS/United States
- UL1 TR001436/TR/NCATS NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UL1 TR002553/TR/NCATS NIH HHS/United States
- UL1 TR002389/TR/NCATS NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- U54 GM104941/GM/NIGMS NIH HHS/United States
- UL1 TR002014/TR/NCATS NIH HHS/United States
- UL1 TR002550/TR/NCATS NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- UL1 TR001855/TR/NCATS NIH HHS/United States
- UL1 TR001425/TR/NCATS NIH HHS/United States
- UL1 TR002373/TR/NCATS NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- UL1 TR002556/TR/NCATS NIH HHS/United States
- UL1 TR003017/TR/NCATS NIH HHS/United States
- UL1 TR001998/TR/NCATS NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- UL1 TR002645/TR/NCATS NIH HHS/United States
- T32 HL139426/HL/NHLBI NIH HHS/United States
- UL1 TR001450/TR/NCATS NIH HHS/United States
- UL1 TR002366/TR/NCATS NIH HHS/United States
- U54 GM115428/GM/NIGMS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- UL1 TR002377/TR/NCATS NIH HHS/United States
- U54 GM115677/GM/NIGMS NIH HHS/United States
- UL1 TR002544/TR/NCATS NIH HHS/United States
- UL1 TR003098/TR/NCATS NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

