In vitro induction of hair follicle signatures using human dermal papilla cells encapsulated in fibrin microgels
- PMID: 37539497
- PMCID: PMC10771113
- DOI: 10.1111/cpr.13528
In vitro induction of hair follicle signatures using human dermal papilla cells encapsulated in fibrin microgels
Abstract
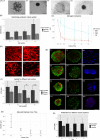
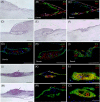
Cellular spheroids have been described as an appropriate culture system to restore human follicle dermal papilla cells (hFDPc) intrinsic properties; however, they show a low and variable efficiency to promote complete hair follicle formation in in vivo experiments. In this work, a conscientious analysis revealed a 25% cell viability in the surface of the dermal papilla spheroid (DPS) for all culture conditions, questioning whether it is an appropriate culture system for hFDPc. To overcome this problem, we propose the use of human blood plasma for the generation of fibrin microgels (FM) with encapsulated hFDPc to restore its inductive signature, either in the presence or in the absence of blood platelets. FM showed a morphology and extracellular matrix composition similar to the native dermal papilla, including Versican and Collagen IV and increasing cell viability up to 85%. While both systems induce epidermal invaginations expressing hair-specific keratins K14, K15, K71, and K75 in in vitro skin cultures, the number of generated structures increases from 17% to 49% when DPS and FM were used, respectively. These data show the potential of our experimental setting for in vitro hair follicle neogenesis with wild adult hFDPc using FM, being a crucial step in the pursuit of human hair follicle regeneration therapies.
© 2023 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Culture and Differentiation of Human Hair Follicle Dermal Papilla Cells in a Soft 3D Self-Assembling Peptide Scaffold.Biomolecules. 2020 Apr 28;10(5):684. doi: 10.3390/biom10050684. Biomolecules. 2020. PMID: 32354097 Free PMC article.
-
Surface Tension Guided Hanging-Drop: Producing Controllable 3D Spheroid of High-Passaged Human Dermal Papilla Cells and Forming Inductive Microtissues for Hair-Follicle Regeneration.ACS Appl Mater Interfaces. 2016 Mar 9;8(9):5906-16. doi: 10.1021/acsami.6b00202. Epub 2016 Feb 29. ACS Appl Mater Interfaces. 2016. PMID: 26886167
-
Wnt activator CHIR99021-stimulated human dermal papilla spheroids contribute to hair follicle formation and production of reconstituted follicle-enriched human skin.Biochem Biophys Res Commun. 2019 Aug 27;516(3):599-605. doi: 10.1016/j.bbrc.2019.06.038. Epub 2019 Jun 18. Biochem Biophys Res Commun. 2019. PMID: 31221480
-
Dermal-epidermal interactions--follicle-derived cell populations in the study of hair-growth mechanisms.J Invest Dermatol. 1993 Jul;101(1 Suppl):33S-38S. doi: 10.1111/1523-1747.ep12362577. J Invest Dermatol. 1993. PMID: 8326152 Review.
-
Maintaining Hair Inductivity in Human Dermal Papilla Cells: A Review of Effective Methods.Skin Pharmacol Physiol. 2020;33(5):280-292. doi: 10.1159/000510152. Epub 2020 Oct 14. Skin Pharmacol Physiol. 2020. PMID: 33053562 Review.
Cited by
-
Hair Regeneration Methods Using Cells Derived from Human Hair Follicles and Challenges to Overcome.Cells. 2024 Dec 25;14(1):7. doi: 10.3390/cells14010007. Cells. 2024. PMID: 39791708 Free PMC article. Review.
-
The role of hsa-miR-193a-5p as an important factor for control of inositol in alopecia areata.Skin Res Technol. 2024 Jul;30(7):e13800. doi: 10.1111/srt.13800. Skin Res Technol. 2024. PMID: 38925555 Free PMC article.
-
Materials-based hair follicle engineering: Basic components and recent advances.Mater Today Bio. 2024 Oct 18;29:101303. doi: 10.1016/j.mtbio.2024.101303. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39498149 Free PMC article. Review.
-
Modelling Human Hair Follicles-Lessons from Animal Models and Beyond.Biology (Basel). 2024 Apr 30;13(5):312. doi: 10.3390/biology13050312. Biology (Basel). 2024. PMID: 38785794 Free PMC article. Review.
References
-
- Couchman JR, Gibson WT. Expression of basement membrane components through morphological changes in the hair growth cycle. Dev Biol. 1985;108(2):290‐298. - PubMed
-
- Messenger AG, Elliott K, Westgate GE, Gibson WT. Distribution of extracellular matrix molecules in human hair follicles. Ann N Y Acad Sci. 1991;642:253‐262. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

