COVID-19 Vaccination in Patients With Cancer and Patients Receiving HSCT or CAR-T Therapy: Immune Response, Real-World Effectiveness, and Implications for the Future
- PMID: 37539765
- PMCID: PMC10401617
- DOI: 10.1093/infdis/jiad174
COVID-19 Vaccination in Patients With Cancer and Patients Receiving HSCT or CAR-T Therapy: Immune Response, Real-World Effectiveness, and Implications for the Future
Abstract
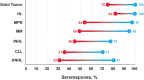
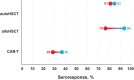
Patients with cancer demonstrate an increased vulnerability for infection and severe disease by SARS-CoV-2, the causative agent of COVID-19. Risk factors for severe COVID-19 include comorbidities, uncontrolled disease, and current line of treatment. Although COVID-19 vaccines have afforded some level of protection against infection and severe disease among patients with solid tumors and hematologic malignancies, decreased immunogenicity and real-world effectiveness have been observed among this population compared with healthy individuals. Characterizing and understanding the immune response to increasing doses or differing schedules of COVID-19 vaccines among patients with cancer is important to inform clinical and public health practices. In this article, we review SARS-CoV-2 susceptibility and immune responses to COVID-19 vaccination in patients with solid tumors, hematologic malignancies, and those receiving hematopoietic stem cell transplant or chimeric-antigen receptor T-cell therapy.
Keywords: chimeric-antigen receptor T-cell therapy; hematologic malignancies; hematopoietic stem cell transplant; mRNA COVID-19 vaccines; solid tumors.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. V. G. H. is supported by a National Health and Medical Research Council postgraduate PhD scholarship (Number 2014210). B. W. T. has participated on advisory boards for Moderna, CSL-Behring, and Takeda, and received grants from Seqirus and MSD, honoraria from Pfizer, Alexion, and Janssen, and is supported by the Australian Government Medical Research Future Fund Fellowship. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Ohm JE, Carbone DP. Immune dysfunction in cancer patients. Oncology (Williston Park) 2002; 16:11–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

