Second messenger signalling bypasses CGRP receptor blockade to provoke migraine attacks in humans
- PMID: 37540009
- PMCID: PMC10690017
- DOI: 10.1093/brain/awad261
Second messenger signalling bypasses CGRP receptor blockade to provoke migraine attacks in humans
Abstract
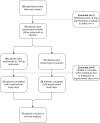
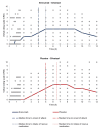
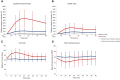
There are several endogenous molecules that can trigger migraine attacks when administered to humans. Notably, calcitonin gene-related peptide (CGRP) has been identified as a key player in a signalling cascade involved in migraine attacks, acting through the second messenger cyclic adenosine monophosphate (cAMP) in various cells, including intracranial vascular smooth muscle cells. However, it remains unclear whether intracellular cAMP signalling requires CGRP receptor activation during a migraine attack in humans. To address this question, we conducted a randomized, double-blind, placebo-controlled, parallel trial using a human provocation model involving the administration of CGRP and cilostazol in individuals with migraine pretreated with erenumab or placebo. Our study revealed that migraine attacks can be provoked in patients by cAMP-mediated mechanisms using cilostazol, even when the CGRP receptor is blocked by erenumab. Furthermore, the dilation of cranial arteries induced by cilostazol was not influenced by the CGRP receptor blockade. These findings provide clinical evidence that cAMP-evoked migraine attacks do not require CGRP receptor activation. This discovery opens up new possibilities for the development of mechanism-based drugs for the treatment of migraine.
Keywords: PACAP; calcitonin; cranial arteries; migraine model; vasodilation.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
M.A. is a consultant, speaker or scientific advisor for AbbVie, Allergan, Amgen, Eli Lilly, Lundbeck, Novartis and Teva, and a primary investigator for ongoing AbbVie/Allergan, Amgen, Eli Lilly, Lundbeck, Novartis and Teva trials. M.A. has no ownership interest and does not own stocks of any pharmaceutical company. M.A. serves as associate editor of
Figures

Comment in
-
CGRP signalling in migraine: time to look downstream?Brain. 2023 Dec 1;146(12):4796-4798. doi: 10.1093/brain/awad390. Brain. 2023. PMID: 37972256 No abstract available.
References
-
- Ashina M, Katsarava Z, Do TP, et al. Migraine: Epidemiology and systems of care. Lancet. 2021;397:1485–1495. - PubMed
-
- Ashina M, Buse DC, Ashina H, et al. Migraine: Integrated approaches to clinical management and emerging treatments. Lancet. 2021;397:1505–1518. - PubMed
-
- Ashina M. Migraine. N Engl J Med. 2020;383:1866–1876. - PubMed
-
- Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 2018;38:1–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

