Angiotensin AT1A receptor signal switching in Agouti-related peptide neurons mediates metabolic rate adaptation during obesity
- PMID: 37540598
- PMCID: PMC10530419
- DOI: 10.1016/j.celrep.2023.112935
Angiotensin AT1A receptor signal switching in Agouti-related peptide neurons mediates metabolic rate adaptation during obesity
Abstract
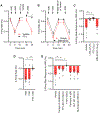
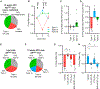
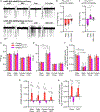
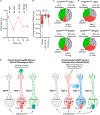
Resting metabolic rate (RMR) adaptation occurs during obesity and is hypothesized to contribute to failed weight management. Angiotensin II (Ang-II) type 1 (AT1A) receptors in Agouti-related peptide (AgRP) neurons contribute to the integrative control of RMR, and deletion of AT1A from AgRP neurons causes RMR adaptation. Extracellular patch-clamp recordings identify distinct cellular responses of individual AgRP neurons from lean mice to Ang-II: no response, inhibition via AT1A and Gαi, or stimulation via Ang-II type 2 (AT2) receptors and Gαq. Following diet-induced obesity, a subset of Ang-II/AT1A-inhibited AgRP neurons undergo a spontaneous G-protein "signal switch," whereby AT1A stop inhibiting the cell via Gαi and instead begin stimulating the cell via Gαq. DREADD-mediated activation of Gαi, but not Gαq, in AT1A-expressing AgRP cells stimulates RMR in lean and obese mice. Thus, loss of AT1A-Gαi coupling within the AT1A-expressing AgRP neuron subtype represents a molecular mechanism contributing to RMR adaptation.
Keywords: CP: Metabolism; CP: Neuroscience; G-protein signaling; arcuate nucleus; obesity; resting metabolic rate.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

