The causal mutation leading to sweetness in modern white lupin cultivars
- PMID: 37540741
- PMCID: PMC10403207
- DOI: 10.1126/sciadv.adg8866
The causal mutation leading to sweetness in modern white lupin cultivars
Abstract
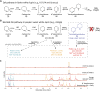
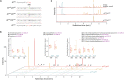
Lupins are high-protein crops that are rapidly gaining interest as hardy alternatives to soybean; however, they accumulate antinutritional alkaloids of the quinolizidine type (QAs). Lupin domestication was enabled by the discovery of genetic loci conferring low QA levels (sweetness), but the precise identity of the underlying genes remains uncertain. We show that pauper, the most common sweet locus in white lupin, encodes an acetyltransferase (AT) unexpectedly involved in the early QA pathway. In pauper plants, a single-nucleotide polymorphism (SNP) strongly impairs AT activity, causing pathway blockage. We corroborate our hypothesis by replicating the pauper chemotype in narrow-leafed lupin via mutagenesis. Our work adds a new dimension to QA biosynthesis and establishes the identity of a lupin sweet gene for the first time, thus facilitating lupin breeding and enabling domestication of other QA-containing legumes.
Figures


References
-
- World Health Organization, Regional Office for Europe, “Plant-based diets and their impact on health, sustainability and the environment: A review of the evidence” (World Health Organization, 2021).
-
- von Sengbusch R., Süßlupinen und Öllupinen, Die Entstehungsgeschichte einiger neuer Kulturpflanzen. Landw. Jahrb. 91, 793–880 (1942).
MeSH terms
LinkOut - more resources
Full Text Sources

