Single-dose mucosal replicon-particle vaccine protects against lethal Nipah virus infection up to 3 days after vaccination
- PMID: 37540755
- PMCID: PMC10403222
- DOI: 10.1126/sciadv.adh4057
Single-dose mucosal replicon-particle vaccine protects against lethal Nipah virus infection up to 3 days after vaccination
Abstract
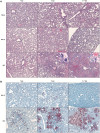
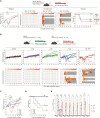
Nipah virus (NiV) causes a highly lethal disease in humans who present with acute respiratory or neurological signs. No vaccines against NiV have been approved to date. Here, we report on the clinical impact of a novel NiV-derived nonspreading replicon particle lacking the fusion (F) protein gene (NiVΔF) as a vaccine in three small animal models of disease. A broad antibody response was detected that included immunoglobulin G (IgG) and IgA subtypes with demonstrable Fc-mediated effector function targeting multiple viral antigens. Single-dose intranasal vaccination up to 3 days before challenge prevented clinical signs and reduced virus levels in hamsters and immunocompromised mice; decreases were seen in tissues and mucosal secretions, critically decreasing potential for virus transmission. This virus replicon particle system provides a vital tool to the field and demonstrates utility as a highly efficacious and safe vaccine candidate that can be administered parenterally or mucosally to protect against lethal Nipah disease.
Figures







References
-
- C. F. Spiropoulou, Nipah virus outbreaks: Still small but extremely lethal. J Infect Dis 219, 1855–1857 (2019). - PubMed
-
- N. I. Paton, Y. S. Leo, S. R. Zaki, A. P. Auchus, K. E. Lee, A. E. Ling, S. K. Chew, B. Ang, P. E. Rollin, T. Umapathi, I. Sng, C. C. Lee, E. Lim, T. G. Ksiazek, Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet 354, 1253–1256 (1999). - PubMed
-
- K. Halpin, A. D. Hyatt, R. Fogarty, D. Middleton, J. Bingham, J. H. Epstein, S. A. Rahman, T. Hughes, C. Smith, H. E. Field, P. Daszak; Henipavirus Ecology Research Group , Pteropid bats are confirmed as the reservoir hosts of henipaviruses: A comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 85, 946–951 (2011). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

