HNRNPC haploinsufficiency affects alternative splicing of intellectual disability-associated genes and causes a neurodevelopmental disorder
- PMID: 37541189
- PMCID: PMC10432175
- DOI: 10.1016/j.ajhg.2023.07.005
HNRNPC haploinsufficiency affects alternative splicing of intellectual disability-associated genes and causes a neurodevelopmental disorder
Abstract
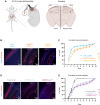
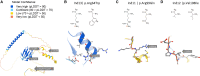
Heterogeneous nuclear ribonucleoprotein C (HNRNPC) is an essential, ubiquitously abundant protein involved in mRNA processing. Genetic variants in other members of the HNRNP family have been associated with neurodevelopmental disorders. Here, we describe 13 individuals with global developmental delay, intellectual disability, behavioral abnormalities, and subtle facial dysmorphology with heterozygous HNRNPC germline variants. Five of them bear an identical in-frame deletion of nine amino acids in the extreme C terminus. To study the effect of this recurrent variant as well as HNRNPC haploinsufficiency, we used induced pluripotent stem cells (iPSCs) and fibroblasts obtained from affected individuals. While protein localization and oligomerization were unaffected by the recurrent C-terminal deletion variant, total HNRNPC levels were decreased. Previously, reduced HNRNPC levels have been associated with changes in alternative splicing. Therefore, we performed a meta-analysis on published RNA-seq datasets of three different cell lines to identify a ubiquitous HNRNPC-dependent signature of alternative spliced exons. The identified signature was not only confirmed in fibroblasts obtained from an affected individual but also showed a significant enrichment for genes associated with intellectual disability. Hence, we assessed the effect of decreased and increased levels of HNRNPC on neuronal arborization and neuronal migration and found that either condition affects neuronal function. Taken together, our data indicate that HNRNPC haploinsufficiency affects alternative splicing of multiple intellectual disability-associated genes and that the developing brain is sensitive to aberrant levels of HNRNPC. Hence, our data strongly support the inclusion of HNRNPC to the family of HNRNP-related neurodevelopmental disorders.
Keywords: HNRNP; HNRNPC; NDD; RNA processing; alternative splicing; heterogeneous ribonucleoprotein C; iPSCs; induced pluripotent stem cells; intellectual disability; neurodevelopmental disorder.
Copyright © 2023 American Society of Human Genetics. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Gillentine M.A., Wang T., Hoekzema K., Rosenfeld J., Liu P., Guo H., Kim C.N., De Vries B.B.A., Vissers L.E.L.M., Nordenskjold M., et al. Rare deleterious mutations of HNRNP genes result in shared neurodevelopmental disorders. Genome Med. 2021;13:63. doi: 10.1186/s13073-021-00870-6. - DOI - PMC - PubMed
-
- Reichert S.C., Li R., A Turner S., van Jaarsveld R.H., Massink M.P.G., van den Boogaard M.J.H., del Toro M., Rodríguez-Palmero A., Fourcade S., Schlüter A., et al. HNRNPH1-related syndromic intellectual disability: Seven additional cases suggestive of a distinct syndromic neurodevelopmental syndrome. Clin. Genet. 2020;98:91–98. doi: 10.1111/cge.13765. - DOI - PubMed
-
- Bain J.M., Cho M.T., Telegrafi A., Wilson A., Brooks S., Botti C., Gowans G., Autullo L.A., Krishnamurthy V., Willing M.C., et al. Variants in HNRNPH2 on the X Chromosome Are Associated with a Neurodevelopmental Disorder in Females. Am. J. Hum. Genet. 2016;99:728–734. doi: 10.1016/j.ajhg.2016.06.028. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

