DRG afferents that mediate physiologic and pathologic mechanosensation from the distal colon
- PMID: 37541195
- PMCID: PMC10440726
- DOI: 10.1016/j.cell.2023.07.007
DRG afferents that mediate physiologic and pathologic mechanosensation from the distal colon
Abstract
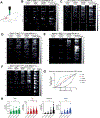
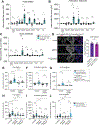
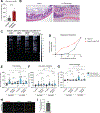
The properties of dorsal root ganglia (DRG) neurons that innervate the distal colon are poorly defined, hindering our understanding of their roles in normal physiology and gastrointestinal (GI) disease. Here, we report genetically defined subsets of colon-innervating DRG neurons with diverse morphologic and physiologic properties. Four colon-innervating DRG neuron populations are mechanosensitive and exhibit distinct force thresholds to colon distension. The highest threshold population, selectively labeled using Bmpr1b genetic tools, is necessary and sufficient for behavioral responses to high colon distension, which is partly mediated by the mechanosensory ion channel Piezo2. This Aδ-HTMR population mediates behavioral over-reactivity to colon distension caused by inflammation in a model of inflammatory bowel disease. Thus, like cutaneous DRG mechanoreceptor populations, colon-innervating mechanoreceptors exhibit distinct anatomical and physiological properties and tile force threshold space, and genetically defined colon-innervating HTMRs mediate pathophysiological responses to colon distension, revealing a target population for therapeutic intervention.
Keywords: colon; dorsal root ganglia; inflammatory pain; mechanosensation; sensory physiology.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
Interoception: Spinal sensory neurons that innervate the intestines.Curr Biol. 2023 Sep 25;33(18):R945-R947. doi: 10.1016/j.cub.2023.07.066. Curr Biol. 2023. PMID: 37751704
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

